当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accessing polysubstituted oxazolidines, pyrrolidines and imidazolidines by regioselective [3 + 2] annulations of ketenimines with donor–acceptor oxiranes and aziridines†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-05-09 00:00:00 , DOI: 10.1039/c8qo00255j Mateo Alajarin 1, 2, 3, 4, 5 , Daniel Bañon 1, 2, 3, 4, 5 , Adrian Egea 1, 2, 3, 4, 5 , Marta Marín-Luna 1, 6, 7, 8 , Raul-Angel Orenes 2, 4, 8, 9, 10 , Angel Vidal 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-05-09 00:00:00 , DOI: 10.1039/c8qo00255j Mateo Alajarin 1, 2, 3, 4, 5 , Daniel Bañon 1, 2, 3, 4, 5 , Adrian Egea 1, 2, 3, 4, 5 , Marta Marín-Luna 1, 6, 7, 8 , Raul-Angel Orenes 2, 4, 8, 9, 10 , Angel Vidal 1, 2, 3, 4, 5
Affiliation
|
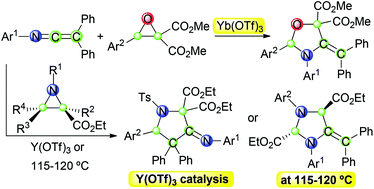
Efficient [3 + 2] annulations of N-aryl-C,C-diphenyl ketenimines with metallo-carbonyl and metallo-azomethine ylides, generated via the respective Yb(OTf)3 and Y(OTf)3 promoted carbon–carbon bond heterolysis of donor–acceptor oxiranes and aziridines, have been accomplished. These reactions proceeded under mild conditions and supplied a general methodology for the regioselective construction of structurally complex oxazolidines and pyrrolidines. Moreover, heating neat mixtures of N-aryl-C,C-diphenyl ketenimines and diethyl aziridine-2,3-dicarboxylates led to imidazolidine derivatives. A computational study concluded in stepwise mechanisms for these [3 + 2] annulations, also shedding light on their regioselectivity, concerning which of the two cumulated double bonds of the ketenimine becomes involved in the reaction with the ylide.
中文翻译:

酮亚胺的区域选择性[3 + 2]环与供体-受体环氧乙烷和氮丙啶的位置接近,获得多取代的恶唑烷,吡咯烷和咪唑烷†
分别通过Yb(OTf)3和Y(OTf)3生成的N-芳基C,C-二苯基酮亚胺与金属羰基和金属偶氮甲亚胺的高效[3 + 2]环化促进了碳的碳-碳键杂合供体-受体环氧乙烷和氮丙啶已完成。这些反应在温和的条件下进行,并为结构复杂的恶唑烷和吡咯烷的区域选择性构建提供了一般方法。而且,加热N-芳基-C,C的纯净混合物-二苯基酮亚胺和二乙基氮丙啶-2,3-二羧酸酯生成咪唑烷衍生物。对这些[3 + 2]环的逐步机理的计算研究得出了结论,并且也揭示了它们的区域选择性,涉及酮亚胺的两个累积双键中的哪一个参与了与叶立德的反应。
更新日期:2018-05-09
中文翻译:

酮亚胺的区域选择性[3 + 2]环与供体-受体环氧乙烷和氮丙啶的位置接近,获得多取代的恶唑烷,吡咯烷和咪唑烷†
分别通过Yb(OTf)3和Y(OTf)3生成的N-芳基C,C-二苯基酮亚胺与金属羰基和金属偶氮甲亚胺的高效[3 + 2]环化促进了碳的碳-碳键杂合供体-受体环氧乙烷和氮丙啶已完成。这些反应在温和的条件下进行,并为结构复杂的恶唑烷和吡咯烷的区域选择性构建提供了一般方法。而且,加热N-芳基-C,C的纯净混合物-二苯基酮亚胺和二乙基氮丙啶-2,3-二羧酸酯生成咪唑烷衍生物。对这些[3 + 2]环的逐步机理的计算研究得出了结论,并且也揭示了它们的区域选择性,涉及酮亚胺的两个累积双键中的哪一个参与了与叶立德的反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号