当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CH3Cl/Cu(410): Interaction and Adsorption Geometry
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acs.jpcc.8b01296 Takamasa Makino 1 , Siti Zulaehah 2 , Jessiel Siaron Gueriba 2 , Wilson Agerico Diño 2, 3 , Michio Okada 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acs.jpcc.8b01296 Takamasa Makino 1 , Siti Zulaehah 2 , Jessiel Siaron Gueriba 2 , Wilson Agerico Diño 2, 3 , Michio Okada 1
Affiliation
|
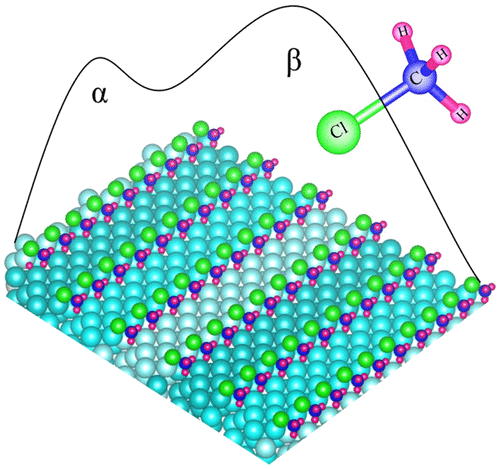
The Rochow process is the most common technology used to prepare organosilicon compounds on an industrial scale, and yet the mechanism is still not well understood. It involves the reaction of methyl chloride (CH3Cl) with silicon, catalyzed by copper. To understand the elementary steps of the reaction involved, we studied the molecular adsorption of CH3Cl/Cu(410) at 100 K and its complete desorption at higher temperatures, 100 K < TD < 200 K. Temperature-programmed desorption (TPD) spectra show two CH3Cl desorption peaks. We attribute the low temperature TPD peak (TD ≈ 120 K) to CH3Cl desorbing from both step-edges and terraces and the high temperature TPD peak (TD ≈ 160 K) to CH3Cl desorbing from the step-edges. Infrared reflection–absorption spectra (IRAS) indicate that at low CH3Cl coverage (Θ = 0.06 ML), CH3Cl adsorbs with its molecular axis (Cl–C bond) aligned either parallel or perpendicular to [001]. At high CH3Cl coverage (Θ ≥ 0.09 ML), CH3Cl adsorbs with its molecular axis aligned perpendicular to [001].
中文翻译:

CH 3 Cl / Cu(410):相互作用和吸附几何
Rochow工艺是用于工业规模制备有机硅化合物的最常用技术,但其机理仍未得到很好的理解。它涉及由铜催化的氯甲烷(CH 3 Cl)与硅的反应。为了了解反应的基本步骤,我们研究了CH 3 Cl / Cu(410)在100 K下的分子吸附以及在100 K < T D <200 K的更高温度下的完全脱附。光谱显示了两个CH 3 Cl解吸峰。我们认为低温TPD峰(Ť d ≈120 K),以CH 3从两个步骤-边缘和台面和高温峰TPD(CL解吸Ť d ≈160 K),以CH 3 CL从工序边缘解吸。红外反射吸收光谱(IRAS)表明,在低CH 3 Cl覆盖率(Θ= 0.06 ML)下,CH 3 Cl的分子轴(Cl-C键)平行或垂直于[001]吸附。在高CH 3 Cl覆盖率(Θ≥0.09 ML)下,CH 3 Cl吸附时其分子轴垂直于[001]。
更新日期:2018-05-08
中文翻译:

CH 3 Cl / Cu(410):相互作用和吸附几何
Rochow工艺是用于工业规模制备有机硅化合物的最常用技术,但其机理仍未得到很好的理解。它涉及由铜催化的氯甲烷(CH 3 Cl)与硅的反应。为了了解反应的基本步骤,我们研究了CH 3 Cl / Cu(410)在100 K下的分子吸附以及在100 K < T D <200 K的更高温度下的完全脱附。光谱显示了两个CH 3 Cl解吸峰。我们认为低温TPD峰(Ť d ≈120 K),以CH 3从两个步骤-边缘和台面和高温峰TPD(CL解吸Ť d ≈160 K),以CH 3 CL从工序边缘解吸。红外反射吸收光谱(IRAS)表明,在低CH 3 Cl覆盖率(Θ= 0.06 ML)下,CH 3 Cl的分子轴(Cl-C键)平行或垂直于[001]吸附。在高CH 3 Cl覆盖率(Θ≥0.09 ML)下,CH 3 Cl吸附时其分子轴垂直于[001]。



























 京公网安备 11010802027423号
京公网安备 11010802027423号