Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-05-08 , DOI: 10.1016/j.bioorg.2018.05.006 Burcu Kilic , Hayrettin O. Gulcan , Fatma Aksakal , Tugba Ercetin , Nihan Oruklu , E. Umit Bagriacik , Deniz S. Dogruer
|
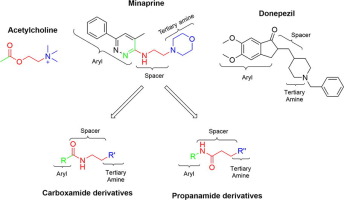
A series of new carboxamide and propanamide derivatives bearing phenylpyridazine as a core ring were designed, synthesized and evaluated for their ability to inhibit both cholinesterase enzymes. In addition, a series of carboxamide and propanamide derivatives bearing biphenyl instead of phenylpyridazine were also synthesized to examine the inhibitory effect of pyridazine moiety on both cholinesterase enzymes. The inhibitory activity results revealed that compounds 5b, 5f, 5h, 5j, 5l pyridazine-3-carboxamide derivative, exhibited selective acetylcholinesterase (AChE) inhibition with IC50 values ranging from 0.11 to 2.69 µM. Among them, compound 5h was the most active one (IC50 = 0.11 µM) without cytotoxic effect at its effective concentration against AChE. Additionally, pyridazine-3-carboxamide derivative 5d (IC50 for AChE = 0.16 µM and IC50 for BChE = 9.80 µM) and biphenyl-4-carboxamide derivative 6d (IC50 for AChE = 0.59 µM and IC50 for BChE = 1.48 µM) displayed dual cholinesterase inhibitory activity. Besides, active compounds were also tested for their ability to inhibit Aβ aggregation. Theoretical physicochemical properties of the compounds were calculated by using Molinspiration Program as well. The Lineweaver-Burk plot and docking study showed that compound 5 h targeted both the catalytic active site (CAS) and the peripheral anionic site (PAS) of AChE.
中文翻译:

一些以苯基哒嗪为核心的羧酰胺和丙酰胺衍生物的设计,合成及其对体外乙酰胆碱酯酶和丁酰胆碱酯酶抑制作用的研究
设计,合成并评估了一系列以苯基哒嗪为核心环的新的羧酰胺和丙酰胺衍生物,以抑制它们同时抑制胆碱酯酶的能力。另外,还合成了一系列带有联苯而不是苯基哒嗪的羧酰胺和丙酰胺衍生物,以检验哒嗪部分对两种胆碱酯酶的抑制作用。抑制活性结果表明,化合物5b,5f,5h,5j,5l哒嗪-3-甲酰胺衍生物表现出选择性乙酰胆碱酯酶(AChE)抑制作用,IC 50值为0.11至2.69 µM。其中,化合物5h是活性最高的化合物(IC 50 = 0.11 µM),且其有效浓度对AChE无细胞毒性作用。此外,哒嗪-3-甲酰胺衍生物5d(AChE的IC 50 = 0.16 µM,BChE的IC 50 = 9.80 µM)和联苯-4-羧酰胺衍生物6d(AChE的IC 50 = 0.59 µM,BChE的IC 50 = 1.48 µM )显示出双重胆碱酯酶抑制活性。此外,还测试了活性化合物抑制Aβ聚集的能力。化合物的理论理化性质也通过使用Molinspiration Program进行计算。Lineweaver-Burk图和对接研究表明,化合物5 h 针对AChE的催化活性位点(CAS)和外围阴离子位点(PAS)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号