当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integration of phospholipid-hyaluronic acid-methotrexate nanocarrier assembly and amphiphilic drug–drug conjugate for synergistic targeted delivery and combinational tumor therapy†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1039/c8bm00009c Yang Li 1, 2, 3, 4, 5 , Huabing Zhang 1, 2, 3, 4, 5 , Yilin Chen 4, 5, 6, 7, 8 , Jinyuan Ma 4, 5, 6, 7, 8 , Jinyan Lin 1, 2, 3, 4, 5 , Yinying Zhang 1, 2, 3, 4, 5 , Zhongxiong Fan 1, 2, 3, 4, 5 , Guanghao Su 8, 9, 10 , Liya Xie 8, 11, 12 , Xuan Zhu 4, 5, 6, 7, 8 , Zhenqing Hou 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1039/c8bm00009c Yang Li 1, 2, 3, 4, 5 , Huabing Zhang 1, 2, 3, 4, 5 , Yilin Chen 4, 5, 6, 7, 8 , Jinyuan Ma 4, 5, 6, 7, 8 , Jinyan Lin 1, 2, 3, 4, 5 , Yinying Zhang 1, 2, 3, 4, 5 , Zhongxiong Fan 1, 2, 3, 4, 5 , Guanghao Su 8, 9, 10 , Liya Xie 8, 11, 12 , Xuan Zhu 4, 5, 6, 7, 8 , Zhenqing Hou 1, 2, 3, 4, 5
Affiliation
|
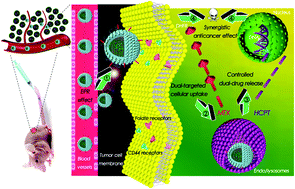
Combinational cancer therapy has been considered as a promising strategy to achieve synergetic therapeutic effects and suppression of multidrug resistance. Herein, we adopted a combination of methotrexate (MTX), an antimetabolite acting on cytoplasm, and 10-hydroxycamptothecin (HCPT), an alkaloid acting on nuclei, to treat cancer. Given the different solubilities, membrane permeabilities, and anticancer mechanisms of both drugs, we developed a dual-targeting delivery system based on 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-hyaluronic acid (a principal ligand of CD44 receptors)-MTX (a selective ligand of folate receptors) nanoparticles, which was exploited to carry HCPT–MTX conjugate for synergistically boosting dual-drug co-delivery. The HCPT–MTX conjugate was synthesized by a blood-stable yet intracellularly hydrolysable ester bond. The core–shell-corona DSPE–HA–MTX nanoparticles encapsulating HCPT–MTX (HCPT–MTX@DHM) exhibited high drug entrapment efficiency (∼91.8%) and pH/esterase-controlled release behavior. Cellular uptake studies confirmed significant increase in the efficiency of selective internalization of HCPT–MTX@DHM via CD44/folate receptors compared with those of DSPE–HA nanoparticles encapsulating HCPT–MTX (HCPT–MTX@DH), both drugs, or each individual drug. Furthermore, in vivo near-infrared fluorescence and photoacoustic dual-modal imaging indicated that DiR-doped HCPT–MTX@DHM nanoparticles efficiently accumulated at the tumor sites through passive-plus-active targeting. Finally, the synergistic active targeting and synchronous dual-drug release at a synergistic drug-to-drug ratio resulted in highly synergetic tumor cell-killing and tumor growth inhibition in MCF-7 tumor-bearing mice. Therefore, HCPT–MTX@DHM nanoparticles can be an efficient and smart platform for tumor-targeting therapy.
中文翻译:

磷脂-透明质酸-甲氨蝶呤纳米载体组件与两亲药物-药物偶联物的整合,用于协同靶向递送和肿瘤联合治疗†
组合癌症疗法已被认为是实现协同治疗效果和抑制多药耐药性的有前途的策略。在这里,我们采用了甲氨蝶呤(MTX)(一种作用于细胞质的抗代谢药)和10-羟基喜树碱(HCPT)(一种作用于细胞核的生物碱)的组合来治疗癌症。考虑到两种药物的溶解度,膜通透性和抗癌机制不同,我们开发了基于1,2-二硬脂酰-sn-甘油的双靶点递送系统-3-磷酸乙醇胺-透明质酸(CD44受体的主要配体)-MTX(叶酸受体的选择性配体)纳米颗粒,用于携带HCPT-MTX共轭物以协同促进双重药物共递送。HCPT-MTX共轭物是通过血液稳定但可在细胞内水解的酯键合成的。包裹HCPT-MTX的核-壳-电晕DSPE-HA-MTX纳米颗粒(HCPT-MTX @ DHM)表现出高的药物截留效率(〜91.8%)和pH /酯酶控制释放行为。细胞摄取研究证实,与包裹HCPT-MTX(HCPT-MTX @ DH),两种药物或每种药物的DSPE-HA纳米颗粒相比,通过CD44 /叶酸受体选择性选择性内化HCPT-MTX @ DHM的效率显着提高。此外,体内近红外荧光和光声双峰成像表明,通过被动加主动靶向,DiR掺杂的HCPT–MTX @ DHM纳米颗粒有效地聚集在肿瘤部位。最后,在具有协同药物与药物比率的情况下,协同主动靶向和同步双重药物释放导致了MCF-7荷瘤小鼠的高度协同肿瘤细胞杀伤和肿瘤生长抑制。因此,HCPT–MTX @ DHM纳米颗粒可以成为肿瘤靶向治疗的有效而智能的平台。
更新日期:2018-05-08
中文翻译:

磷脂-透明质酸-甲氨蝶呤纳米载体组件与两亲药物-药物偶联物的整合,用于协同靶向递送和肿瘤联合治疗†
组合癌症疗法已被认为是实现协同治疗效果和抑制多药耐药性的有前途的策略。在这里,我们采用了甲氨蝶呤(MTX)(一种作用于细胞质的抗代谢药)和10-羟基喜树碱(HCPT)(一种作用于细胞核的生物碱)的组合来治疗癌症。考虑到两种药物的溶解度,膜通透性和抗癌机制不同,我们开发了基于1,2-二硬脂酰-sn-甘油的双靶点递送系统-3-磷酸乙醇胺-透明质酸(CD44受体的主要配体)-MTX(叶酸受体的选择性配体)纳米颗粒,用于携带HCPT-MTX共轭物以协同促进双重药物共递送。HCPT-MTX共轭物是通过血液稳定但可在细胞内水解的酯键合成的。包裹HCPT-MTX的核-壳-电晕DSPE-HA-MTX纳米颗粒(HCPT-MTX @ DHM)表现出高的药物截留效率(〜91.8%)和pH /酯酶控制释放行为。细胞摄取研究证实,与包裹HCPT-MTX(HCPT-MTX @ DH),两种药物或每种药物的DSPE-HA纳米颗粒相比,通过CD44 /叶酸受体选择性选择性内化HCPT-MTX @ DHM的效率显着提高。此外,体内近红外荧光和光声双峰成像表明,通过被动加主动靶向,DiR掺杂的HCPT–MTX @ DHM纳米颗粒有效地聚集在肿瘤部位。最后,在具有协同药物与药物比率的情况下,协同主动靶向和同步双重药物释放导致了MCF-7荷瘤小鼠的高度协同肿瘤细胞杀伤和肿瘤生长抑制。因此,HCPT–MTX @ DHM纳米颗粒可以成为肿瘤靶向治疗的有效而智能的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号