当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MoS2 Quantum Dots: Effect of Hydrogenation on Surface Stability and H2S Release
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-05-21 , DOI: 10.1021/acs.jpcc.8b04198 Prashant P. Shinde 1 , Shashishekar P. Adiga 1 , Shanthi Pandian 1 , Sanoop Ramachandran 1 , Krishnan S. Hariharan 1 , Subramanya M. Kolake 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-05-21 , DOI: 10.1021/acs.jpcc.8b04198 Prashant P. Shinde 1 , Shashishekar P. Adiga 1 , Shanthi Pandian 1 , Sanoop Ramachandran 1 , Krishnan S. Hariharan 1 , Subramanya M. Kolake 1
Affiliation
|
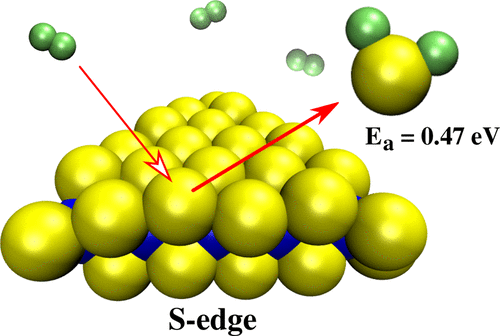
We employ density functional theory to investigate effects of hydrogenation on the energetic stability and electronic properties of triangular MoS2 nanoclusters with S-edges. Excess edge sulfur atoms relative to the bulk stoichiometry along the edges are passivated by hydrogen atoms. We find that the hydrogen coverage for maximum stability can be calculated by (n – 2)/2(n – 1), where n is the number of S atoms along an edge. The energetics reveal a preference for the zigzag arrangement of adsorbed hydrogen atoms on the edges. Our calculations show vanishing HOMO–LUMO gaps mainly due to the presence of dangling bonds at the edges and can be considered metal-like. We find that the activation energy required to release H2S lies in between 0.47 and 0.62 eV, and this value is in good agreement with the recently reported experimental value.
中文翻译:

MoS 2量子点:加氢对表面稳定性和H 2 S释放的影响
我们采用密度泛函理论来研究氢化对具有S边的三角形MoS 2纳米团簇的能量稳定性和电子性质的影响。相对于沿边缘的整体化学计量的过量边缘硫原子被氢原子钝化。我们发现,可以通过(n – 2)/ 2(n – 1)计算最大稳定性的氢覆盖率,其中n是沿边缘的S原子数。高能学揭示了对边缘上吸附的氢原子的锯齿形排列的偏爱。我们的计算结果表明,HOMO-LUMO间隙逐渐消失,这主要是由于边缘处存在悬空键,可以将其视为类金属。我们发现释放H 2所需的活化能S在0.47至0.62 eV之间,该值与最近报道的实验值非常吻合。
更新日期:2019-11-18
中文翻译:

MoS 2量子点:加氢对表面稳定性和H 2 S释放的影响
我们采用密度泛函理论来研究氢化对具有S边的三角形MoS 2纳米团簇的能量稳定性和电子性质的影响。相对于沿边缘的整体化学计量的过量边缘硫原子被氢原子钝化。我们发现,可以通过(n – 2)/ 2(n – 1)计算最大稳定性的氢覆盖率,其中n是沿边缘的S原子数。高能学揭示了对边缘上吸附的氢原子的锯齿形排列的偏爱。我们的计算结果表明,HOMO-LUMO间隙逐渐消失,这主要是由于边缘处存在悬空键,可以将其视为类金属。我们发现释放H 2所需的活化能S在0.47至0.62 eV之间,该值与最近报道的实验值非常吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号