Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-05-07 , DOI: 10.1016/j.bioorg.2018.05.003 Adebayo Tajudeen Bale , Khalid Mohammed Khan , Uzma Salar , Sridevi Chigurupati , Tolulope Fasina , Farman Ali , Kanwal , Abdul Wadood , Muhammad Taha , Sitansu Sekhar Nanda , Mehreen Ghufran , Shahnaz Perveen
|
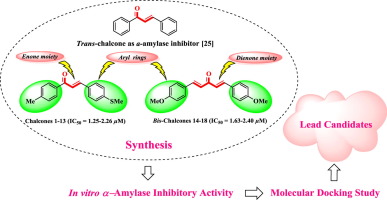
Despite of a diverse range of biological activities associated with chalcones and bis-chalcones, they are still neglected by the medicinal chemist for their possible α-amylase inhibitory activity. So, the current study is based on the evaluation of this class for the identification of new leads as α-amylase inhibitors. For that purpose, a library of substituted chalcones 1–13 and bis-chalcones 14–18 were synthesized and characterized by spectroscopic techniques EI-MS and 1H NMR. CHN analysis was carried out and found in agreement with the calculated values. All compounds were evaluated for in vitro α-amylase inhibitory activity and demonstrated good activities in the range of IC50 = 1.25 ± 1.05–2.40 ± 0.09 µM as compared to the standard acarbose (IC50 = 1.04 ± 0.3 µM). Limited structure–activity relationship (SAR) was established by considering the effect of different groups attached to aryl rings on varying inhibitory activity. SMe group in chalcones and OMe group in bis-chalcones were found more influential on the activity than other groups. However, in order to predict the involvement of different groups in the binding interactions with the active site of α-amylase enzyme, in silico studies were also conducted.
中文翻译:

查耳酮和双查耳酮:作为潜在的α-淀粉酶抑制剂;合成,体外筛选和分子模型研究
尽管与查耳酮和双查耳酮有关的生物活性范围很广,但由于可能的α-淀粉酶抑制活性,它们仍被药物化学家忽略。因此,当前的研究是基于对此类的评估,以鉴定出新的铅作为α-淀粉酶抑制剂。为了这个目的,取代的查耳酮的文库1 - 13和双-chalcones 14 - 18通过光谱技术EI-MS和合成和表征1。1H NMR 进行了CHN分析,并与计算值相符。对所有化合物进行了评估 与标准阿卡波糖(IC 50 = 1.04±0.3 µM)相比,具有体外α-淀粉酶抑制活性,并且在IC 50 = 1.25±1.05–2.40±0.09 µM范围内表现出良好的活性。通过考虑与芳基环相连的不同基团对不同抑制活性的影响,建立了有限的结构-活性关系(SAR)。发现查耳酮的SMe组和双查耳酮的OMe组比其他组对活性有更大的影响。然而,为了预测不同基团参与与α-淀粉酶活性位点的结合相互作用,还进行了计算机研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号