Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2018-05-06 , DOI: 10.1016/j.apcatb.2018.05.016 Rudy Crisafulli , Vanine V. Silva de Barros , Francisca E. Rodrigues de Oliveira , Thairo de Araújo Rocha , Sabrina Zignani , Lorenzo Spadaro , Alessandra Palella , José A. Dias , José J. Linares
|
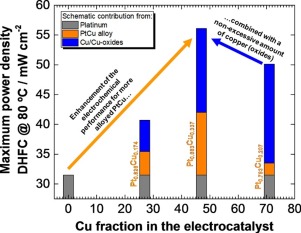
Pt/C and PtCu/C electrocatalysts with nominal Pt:Cu atomic ratios of 75:25, 50:50, and 25:75 were prepared using N2H4 as reducing agent and carbon black Vulcan XC-72R as support. The obtained materials were physically characterized by X-ray diffraction, Energy-Dispersive X-ray analysis, Transmission Electron Microscopy images, X-ray Photoelectron Spectroscopy (XPS), and Temperature-Programmed Reduction analysis. Cyclic voltammetry, linear sweep voltammetry, and chronoamperometry (TPR) measurements were carried out in a three-electrode glass cell to evaluate the electrochemical activity towards hydrazine electrooxidation in alkaline medium along with single-cell direct hydrazine fuel cell (DHFC) tests. The actual composition of the electrocatalysts evidenced a slightly lower Cu fraction compared to the nominal one. The X-ray diffractograms of the electrocatalysts showed the typical face-centered cubic structure of Pt alloys, with the highest fraction of Cu alloyed to Pt being achieved with the almost equiatomic catalyst. An important fraction of the remaining non-alloyed Cu is in the form of a copper oxide, as evidenced by XPS and TPR measurements. The electrochemical tests evidenced that the coexistence of part of the Cu alloyed with Pt and copper oxide achieved in the PtCu/C electrocatalysts enhances the performance compared to Pt/C. In particular, the optimum formulation is attained by the Pt53Cu47/C electrocatalyst, allowing maximization of the electrocatalytic activity towards hydrazine electrooxidation and the single-cell performance at 60 and 80 °C.
中文翻译:

碱性介质中铜对铂促进肼电氧化的促进作用
使用N 2 H 4制备标称Pt:Cu原子比为75:25、50:50和25:75的Pt / C和PtCu / C电催化剂作为还原剂,炭黑Vulcan XC-72R作为载体。通过X射线衍射,能量色散X射线分析,透射电子显微镜图像,X射线光电子能谱(XPS)和程序升温还原分析对所得材料进行物理表征。在三电极玻璃电池中进行了循环伏安法,线性扫描伏安法和计时安培法(TPR)测量,以评估碱性介质中对肼电氧化的电化学活性以及单电池直接肼燃料电池(DHFC)测试。与标称催化剂相比,电催化剂的实际组成表明其铜含量略低。电催化剂的X射线衍射图显示了Pt合金的典型面心立方结构,几乎等原子的催化剂可实现与Pt合金化的最高含量的Cu。XPS和TPR测量表明,剩余的非合金Cu的重要部分为氧化铜形式。电化学测试表明,与Pt / C相比,在PtCu / C电催化剂中实现的与Pt和氧化铜合金化的部分Cu的共存增强了性能。特别地,通过Pt获得最佳配方53 Cu 47 / C电催化剂,可在60和80°C下最大限度地提高对肼电氧化的电催化活性和单电池性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号