当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tenascin-C derived signaling induces neuronal differentiation in a three-dimensional peptide nanofiber gel†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1039/c7bm00850c Melike Sever 1, 2, 3, 4, 5 , Gokhan Gunay 3, 4, 5, 6 , Mustafa O. Guler 7, 8, 9, 10 , Ayse B. Tekinay 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1039/c7bm00850c Melike Sever 1, 2, 3, 4, 5 , Gokhan Gunay 3, 4, 5, 6 , Mustafa O. Guler 7, 8, 9, 10 , Ayse B. Tekinay 1, 2, 3, 4, 5
Affiliation
|
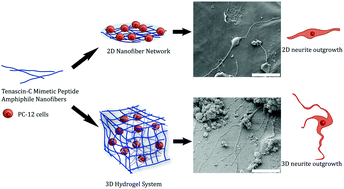
The development of new biomaterials mimicking the neuronal extracellular matrix (ECM) requires signals for the induction of neuronal differentiation and regeneration. In addition to the biological and chemical cues, the physical properties of the ECM should also be considered while designing regenerative materials for nervous tissue. In this study, we investigated the influence of the microenvironment on tenascin-C signaling using 2D surfaces and 3D scaffolds generated by a peptide amphiphile nanofiber gel with a tenascin-C derived peptide epitope (VFDNFVLK). While tenascin-C mimetic PA nanofibers significantly increased the length and number of neurites produced by PC12 cells on 2D cell culture, more extensive neurite outgrowth was observed in the 3D gel environment. PC12 cells encapsulated within the 3D tenascin-C mimetic peptide nanofiber gel also exhibited significantly increased expression of neural markers compared to the cells on 2D surfaces. Our results emphasize the synergistic effects of the 3D conformation of peptide nanofibers along with the tenascin-C signaling and growth factors on the neuronal differentiation of PC12 cells, which may further provide more tissue-like morphology for therapeutic applications.
中文翻译:

肌腱蛋白C衍生的信号传导在三维肽纳米纤维凝胶中诱导神经元分化†
模仿神经元细胞外基质(ECM)的新生物材料的开发需要用于诱导神经元分化和再生的信号。除了生物学和化学提示外,在设计神经组织再生材料时还应考虑ECM的物理特性。在这项研究中,我们调查了微环境对腱糖蛋白C信号的影响,使用2D表面和3D支架,该表面由具有肽蛋白两亲性纳米纤维凝胶和腱糖蛋白C衍生的肽表位(VFDNFVLK)生成。尽管腱生蛋白-C模拟PA纳米纤维显着增加了PC12细胞在2D细胞培养中产生的神经突的长度和数量,但在3D凝胶环境中观察到了更广泛的神经突向外生长。与2D表面上的细胞相比,封装在3D腱生蛋白-C模拟肽纳米纤维凝胶中的PC12细胞还表现出神经标志物的表达显着增加。我们的结果强调了肽纳米纤维的3D构象以及腱生蛋白C信号传导和生长因子对PC12细胞神经元分化的协同作用,这可能进一步为治疗应用提供更多的组织样形态。
更新日期:2018-05-02
中文翻译:

肌腱蛋白C衍生的信号传导在三维肽纳米纤维凝胶中诱导神经元分化†
模仿神经元细胞外基质(ECM)的新生物材料的开发需要用于诱导神经元分化和再生的信号。除了生物学和化学提示外,在设计神经组织再生材料时还应考虑ECM的物理特性。在这项研究中,我们调查了微环境对腱糖蛋白C信号的影响,使用2D表面和3D支架,该表面由具有肽蛋白两亲性纳米纤维凝胶和腱糖蛋白C衍生的肽表位(VFDNFVLK)生成。尽管腱生蛋白-C模拟PA纳米纤维显着增加了PC12细胞在2D细胞培养中产生的神经突的长度和数量,但在3D凝胶环境中观察到了更广泛的神经突向外生长。与2D表面上的细胞相比,封装在3D腱生蛋白-C模拟肽纳米纤维凝胶中的PC12细胞还表现出神经标志物的表达显着增加。我们的结果强调了肽纳米纤维的3D构象以及腱生蛋白C信号传导和生长因子对PC12细胞神经元分化的协同作用,这可能进一步为治疗应用提供更多的组织样形态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号