当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Oxidation of Peroxide by Disordered Metal Oxides: A Measurement of Thermodynamic Stability "By Proxy".
ChemPlusChem ( IF 3.4 ) Pub Date : 2018-05-18 , DOI: 10.1002/cplu.201800150 Mayada Sabri 1, 2, 3 , Hannah J King 1, 2 , Rosalind J Gummow 2 , François Malherbe 1 , Rosalie K Hocking 1, 2
ChemPlusChem ( IF 3.4 ) Pub Date : 2018-05-18 , DOI: 10.1002/cplu.201800150 Mayada Sabri 1, 2, 3 , Hannah J King 1, 2 , Rosalind J Gummow 2 , François Malherbe 1 , Rosalie K Hocking 1, 2
Affiliation
|
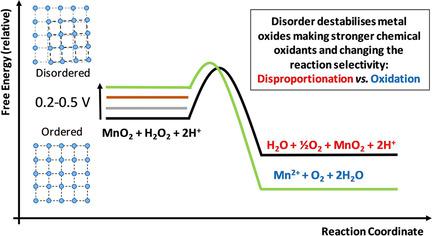
It is often noted that disordered materials have different chemical properties to their more "ordered" cousins. Quantifying these effects in terms of thermodynamics is challenging in part because disordered materials can be difficult to characterise and are frequently relatively unstable. During the course of our experiments to understand the effects of disorder in catalysts for water oxidation we observed that many disordered manganese and cobalt oxide water oxidation catalysts directly oxidised peroxide in contrast to their more ordered analogues which catalysed its disproportionation, that is, MnO2 +2 H+ +H2 O2 →Mn2+ +2 H2 O+O2 (oxidation) versus H2 O2 →H2 O+ 1 / 2 O2 (disproportionation). By measuring the efficiency for one reaction over the other as a function of pH, we were able to quantify the relative stability of materials in two series of metal oxides and thereby quantify their relative thermodynamic stability, "by proxy". We found that for the series of catalysts investigated the disorder made the materials stronger chemical oxidants and worse catalysts for the disproportionation of peroxide.
中文翻译:

无序金属氧化物对过氧化物的氧化:热力学稳定性的“代用”测量。
经常注意到,无序材料与其更“有序”的表亲具有不同的化学性质。从热力学角度量化这些影响具有挑战性,部分原因是无序的材料可能难以表征且通常相对不稳定。在了解水氧化催化剂中无序影响的实验过程中,我们观察到许多无序的锰和钴氧化物水氧化催化剂直接氧化了过氧化物,与其催化有歧化作用的更类似的类似物(即MnO2 +2)形成了对比。 H + + H2 O2→Mn2 + +2 H2 O + O2(氧化)相对于H2 O2→H2 O + 1/2 O2(歧化)。通过测量一个反应相对于另一个反应的效率随pH的变化,我们能够通过“代理”来量化两个系列金属氧化物中材料的相对稳定性,从而量化它们的相对热力学稳定性。我们发现,对于所研究的一系列催化剂,无序状态使材料变得更强的化学氧化剂,而使过氧化物歧化的催化剂变差。
更新日期:2018-05-18
中文翻译:

无序金属氧化物对过氧化物的氧化:热力学稳定性的“代用”测量。
经常注意到,无序材料与其更“有序”的表亲具有不同的化学性质。从热力学角度量化这些影响具有挑战性,部分原因是无序的材料可能难以表征且通常相对不稳定。在了解水氧化催化剂中无序影响的实验过程中,我们观察到许多无序的锰和钴氧化物水氧化催化剂直接氧化了过氧化物,与其催化有歧化作用的更类似的类似物(即MnO2 +2)形成了对比。 H + + H2 O2→Mn2 + +2 H2 O + O2(氧化)相对于H2 O2→H2 O + 1/2 O2(歧化)。通过测量一个反应相对于另一个反应的效率随pH的变化,我们能够通过“代理”来量化两个系列金属氧化物中材料的相对稳定性,从而量化它们的相对热力学稳定性。我们发现,对于所研究的一系列催化剂,无序状态使材料变得更强的化学氧化剂,而使过氧化物歧化的催化剂变差。


























 京公网安备 11010802027423号
京公网安备 11010802027423号