当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Divergent Arylation of Triazolopyridines: A Computational Study
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-06-20 , DOI: 10.1002/asia.201800498 Deyaa I. AbuSalim 1 , Sungwoo Hong 1, 2 , Mu-Hyun Baik 1, 2
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-06-20 , DOI: 10.1002/asia.201800498 Deyaa I. AbuSalim 1 , Sungwoo Hong 1, 2 , Mu-Hyun Baik 1, 2
Affiliation
|
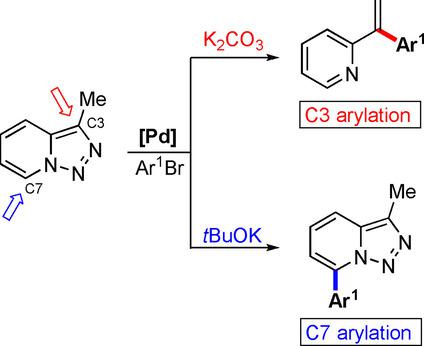
The mechanisms for new palladium‐catalyzed divergent reactions of triazolopyridines were investigated by means of DFT calculations. Previously, it was observed experimentally that cross‐coupling at the C7‐position of triazolopyridines occurred when a strong base was used, whereas the reaction could be diverted to the C3‐position if a weak base was employed. Calculations suggest that a strong base, such as tert‐butoxide, can easily deprotonate C7−H, independent of the palladium metal, and deliver the preactivated substrate to palladium, which can reductively eliminate the final product. Without a strong base, the palladium(II) center reacts with the ring‐opened diazo imine isomer of triazolopyridine to initially form a palladium(II)–carbene intermediate, which undergoes migratory insertion followed by β‐hydride elimination to afford a 1,1‐disubstituted alkene.
中文翻译:

钯催化的三唑并吡啶的发散丙烯酸化:计算研究
通过DFT计算研究了新的钯催化的三唑并吡啶的发散反应的机理。以前,通过实验观察到,当使用强碱时,三唑并吡啶的C7位发生交叉偶联,而如果使用弱碱,则该反应可转移至C3位。计算结果表明,有很强的基础,例如叔叔丁醇盐可以很容易地使C7–H质子化,而与钯金属无关,并将预活化的底物传递到钯上,从而可以还原性地消除最终产物。在没有强碱的情况下,钯(II)中心与三唑并吡啶的开环重氮亚胺异构体反应,最初形成钯(II)-卡宾中间体,该中间体经过迁移插入,然后经β-氢化物消除,得到1,1。 -二取代的烯烃。
更新日期:2018-06-20
中文翻译:

钯催化的三唑并吡啶的发散丙烯酸化:计算研究
通过DFT计算研究了新的钯催化的三唑并吡啶的发散反应的机理。以前,通过实验观察到,当使用强碱时,三唑并吡啶的C7位发生交叉偶联,而如果使用弱碱,则该反应可转移至C3位。计算结果表明,有很强的基础,例如叔叔丁醇盐可以很容易地使C7–H质子化,而与钯金属无关,并将预活化的底物传递到钯上,从而可以还原性地消除最终产物。在没有强碱的情况下,钯(II)中心与三唑并吡啶的开环重氮亚胺异构体反应,最初形成钯(II)-卡宾中间体,该中间体经过迁移插入,然后经β-氢化物消除,得到1,1。 -二取代的烯烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号