当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
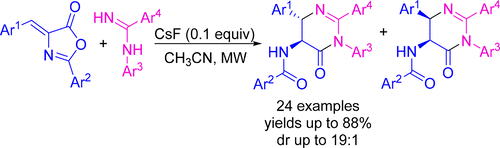
CsF-Catalyzed Transannulation Reaction of Oxazolones: Diastereoselective Synthesis of Diversified trans-N-(6-Oxo-1,4,5,6-tetrahydropyrimidin-5-yl)benzamides with Arylidene Azlactones and Amidines
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1021/acscombsci.8b00027 Golnaz Parhizkar 1 , Ahmad Reza Khosropour 1 , Iraj Mohammadpoor-Baltork 1 , Elahehnaz Parhizkar 2 , Hadi Amiri Rudbari 1
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1021/acscombsci.8b00027 Golnaz Parhizkar 1 , Ahmad Reza Khosropour 1 , Iraj Mohammadpoor-Baltork 1 , Elahehnaz Parhizkar 2 , Hadi Amiri Rudbari 1
Affiliation
|
A versatile and straightforward synthetic strategy for the construction of new tetrasubstituted 1,3-diazinones is described. The procedure is based on CsF-catalyzed, microwave-assisted, ring transformation reaction of arylidene azlactones with amidines. Moreover, this technique provides diversified trans-N-(6-oxo-1,4,5,6-tetrahydropyrimidin-5-yl)benzamides with a good antimicrobial activity.
中文翻译:

恶唑酮的CSF-催化Transannulation反应:多种的选择性合成反式- ñ - (6-氧代-1,4,5,6-四氢嘧啶-5-基)苯甲酰胺与芳基亚甲基吖内酯和脒
描述了一种用于构建新的四取代的1,3-二氮杂二酮的通用且直接的合成策略。该步骤基于CsF催化的亚芳基z内酯与am的微波辅助环转化反应。此外,该技术提供了多元化反式- ñ - (6-氧代-1,4,5,6-四氢嘧啶-5-基)具有良好的抗微生物活性的苯甲酰胺。
更新日期:2018-04-24
中文翻译:

恶唑酮的CSF-催化Transannulation反应:多种的选择性合成反式- ñ - (6-氧代-1,4,5,6-四氢嘧啶-5-基)苯甲酰胺与芳基亚甲基吖内酯和脒
描述了一种用于构建新的四取代的1,3-二氮杂二酮的通用且直接的合成策略。该步骤基于CsF催化的亚芳基z内酯与am的微波辅助环转化反应。此外,该技术提供了多元化反式- ñ - (6-氧代-1,4,5,6-四氢嘧啶-5-基)具有良好的抗微生物活性的苯甲酰胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号