当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical insights on the inhibition mechanism of a class A Serine Hydrolase by avibactam
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2018-04-29 , DOI: 10.1002/jcc.25340 Ignacio Lizana 1 , Eduardo J. Delgado 1
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2018-04-29 , DOI: 10.1002/jcc.25340 Ignacio Lizana 1 , Eduardo J. Delgado 1
Affiliation
|
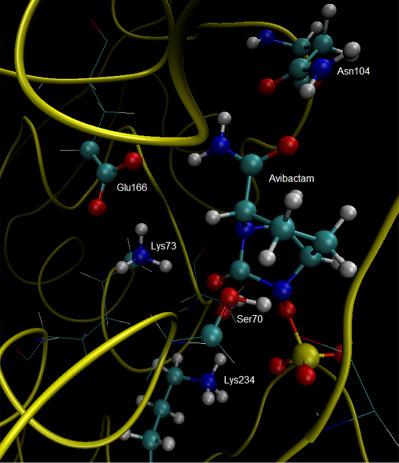
The inhibition mechanism of CTX‐M‐15 class A serine hydrolase by the inhibitor avibactam is addressed by a combined molecular dynamics (MD) and quantum mechanics/molecular mechanics (QM/MM) approach postulating that the residue Ser70 is the sole reacting residue, that is, itself may play the role of the acid–base species required for the enzyme inhibition. Other residues located in the active site have key participation in the positioning of the inhibitor in the right conformation to favor the attack of Ser70, in addition to the stabilization of the transition state by electrostatic interactions with avibactam. The results validate the hypothesis and show that the reaction follows an asynchronous concerted mechanism, in which the nucleophilic attack of the hydroxyl oxygen of Ser70 precedes the protonation of the amidic nitrogen and ring opening. The calculated activation barrier is 16 kcal/mol in agreement with the experimental evidence. © 2018 Wiley Periodicals, Inc.
中文翻译:

阿维巴坦抑制A类丝氨酸水解酶机制的理论见解
抑制剂阿维巴坦对 CTX-M-15 A 类丝氨酸水解酶的抑制机制通过结合分子动力学 (MD) 和量子力学/分子力学 (QM/MM) 方法来解决,假设残基 Ser70 是唯一的反应残基,也就是说,它本身可能起到抑制酶所需的酸碱物质的作用。除了通过与 avibactam 的静电相互作用稳定过渡态之外,位于活性位点的其他残基还关键参与了将抑制剂定位在正确构象中以有利于 Ser70 的攻击。结果验证了这一假设,并表明该反应遵循异步协调机制,其中 Ser70 的羟基氧的亲核攻击先于酰胺氮的质子化和开环。计算出的活化势垒为 16 kcal/mol,与实验证据一致。© 2018 Wiley Periodicals, Inc.
更新日期:2018-04-29
中文翻译:

阿维巴坦抑制A类丝氨酸水解酶机制的理论见解
抑制剂阿维巴坦对 CTX-M-15 A 类丝氨酸水解酶的抑制机制通过结合分子动力学 (MD) 和量子力学/分子力学 (QM/MM) 方法来解决,假设残基 Ser70 是唯一的反应残基,也就是说,它本身可能起到抑制酶所需的酸碱物质的作用。除了通过与 avibactam 的静电相互作用稳定过渡态之外,位于活性位点的其他残基还关键参与了将抑制剂定位在正确构象中以有利于 Ser70 的攻击。结果验证了这一假设,并表明该反应遵循异步协调机制,其中 Ser70 的羟基氧的亲核攻击先于酰胺氮的质子化和开环。计算出的活化势垒为 16 kcal/mol,与实验证据一致。© 2018 Wiley Periodicals, Inc.


























 京公网安备 11010802027423号
京公网安备 11010802027423号