当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual-protected amino acid derivatives as new antitubercular agents.
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-05-31 , DOI: 10.1111/cbdd.13315 Pedro P de Castro 1 , Débora L Campos 2 , Fernando R Pavan 2 , Giovanni W Amarante 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-05-31 , DOI: 10.1111/cbdd.13315 Pedro P de Castro 1 , Débora L Campos 2 , Fernando R Pavan 2 , Giovanni W Amarante 1
Affiliation
|
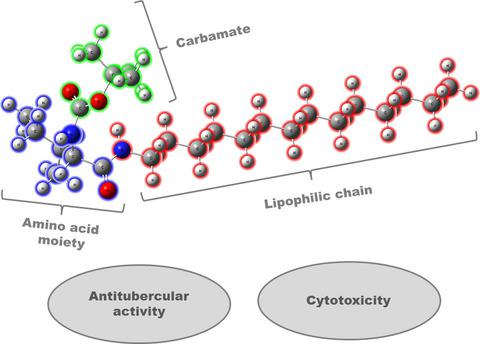
Tuberculosis is an infectious disease with high incidence and growing drug-resistant rates. In an attempt to develop new antitubercular agents, 35 compounds were synthesized, most of them bearing a carbamate and enantiopure amino acid moiety. These compounds had their activity evaluated toward a Mycobacterium tuberculosis strain (ATCC 27294) and cytotoxicity against fibroblast MRC-5 cells (ATCC CCL-171). Three of the prepared derivatives presented a good antimicrobial inhibition and two of them a moderate cytotoxicity. The lipophilicity seems to play a vital role in the cell growth activity, with best results for the derivatives with a higher logP.
中文翻译:

双重保护的氨基酸衍生物作为新型抗结核药。
结核病是一种传染病,发病率高,耐药率不断上升。为了开发新的抗结核药,合成了35种化合物,其中大多数带有氨基甲酸酯和对映体纯氨基酸部分。评估了这些化合物对结核分枝杆菌菌株的活性(ATCC 27294)和对成纤维细胞MRC-5细胞的细胞毒性(ATCC CCL-171)。所制备的三种衍生物具有良好的抗菌抑制作用,其中两种具有中等的细胞毒性。亲脂性似乎在细胞生长活性中起着至关重要的作用,对于具有较高logP的衍生物而言,其效果最佳。
更新日期:2018-05-31
中文翻译:

双重保护的氨基酸衍生物作为新型抗结核药。
结核病是一种传染病,发病率高,耐药率不断上升。为了开发新的抗结核药,合成了35种化合物,其中大多数带有氨基甲酸酯和对映体纯氨基酸部分。评估了这些化合物对结核分枝杆菌菌株的活性(ATCC 27294)和对成纤维细胞MRC-5细胞的细胞毒性(ATCC CCL-171)。所制备的三种衍生物具有良好的抗菌抑制作用,其中两种具有中等的细胞毒性。亲脂性似乎在细胞生长活性中起着至关重要的作用,对于具有较高logP的衍生物而言,其效果最佳。



























 京公网安备 11010802027423号
京公网安备 11010802027423号