当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Insight Into the EC’ Mechanism of Uric Acid Regeneration in the Presence of Ascorbic Acid on a Poly(3,4-ethylenedioxithiophene) Modified Gold Electrode
Electroanalysis ( IF 3 ) Pub Date : 2018-03-23 , DOI: 10.1002/elan.201800024 Cédric Crosnier de Lassichere 1 , Laure Latapie 2 , David Evrard 2 , Pierre Gros 2
Electroanalysis ( IF 3 ) Pub Date : 2018-03-23 , DOI: 10.1002/elan.201800024 Cédric Crosnier de Lassichere 1 , Laure Latapie 2 , David Evrard 2 , Pierre Gros 2
Affiliation
|
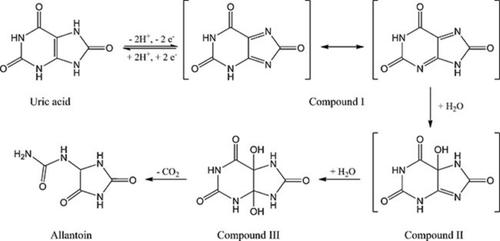
A gold electrode surface was functionalized by means of an electropolymerized conductive poly(3,4-ethylenedioxythiophene) (PEDOT) organic layer. This modified electrode was used for the electrochemical detection of ascorbic (AA) and uric (UA) acids in an aqueous mixture with a selectivity around 340 mV. The electrochemical reactions kinetics were limited by AA diffusion and UA adsorption at the electrode surface, respectively. Following a previous study ([Electrochem Comm., 2011, 13, 423-425]) cyclic voltammetry was used to provide a better understanding of the EC' mechanism of regeneration of UA by AA. Experiments particularly showed that allantoin (i.e. the final product of UA oxidation) is not actually involved in the synergic mechanism but rather the oxidized UA product diimine which is adsorbed at the electrode surface.
中文翻译:

聚(3,4-亚乙基二氧噻吩)修饰的金电极上抗坏血酸存在下尿酸再生的EC机制的新见解
金电极表面通过电聚合导电聚(3,4-亚乙基二氧噻吩)(PEDOT)有机层功能化。该修饰电极用于电化学检测水性混合物中的抗坏血酸 (AA) 和尿酸 (UA),选择性约为 340 mV。电化学反应动力学分别受到电极表面 AA 扩散和 UA 吸附的限制。根据先前的研究 ([Electrochem Comm., 2011, 13, 423-425]),使用循环伏安法更好地了解 EC' AA 再生 UA 的机制。实验特别表明尿囊素(即 UA 氧化的最终产物)实际上并未参与协同机制,而是吸附在电极表面的氧化 UA 产物二亚胺。
更新日期:2018-03-23
中文翻译:

聚(3,4-亚乙基二氧噻吩)修饰的金电极上抗坏血酸存在下尿酸再生的EC机制的新见解
金电极表面通过电聚合导电聚(3,4-亚乙基二氧噻吩)(PEDOT)有机层功能化。该修饰电极用于电化学检测水性混合物中的抗坏血酸 (AA) 和尿酸 (UA),选择性约为 340 mV。电化学反应动力学分别受到电极表面 AA 扩散和 UA 吸附的限制。根据先前的研究 ([Electrochem Comm., 2011, 13, 423-425]),使用循环伏安法更好地了解 EC' AA 再生 UA 的机制。实验特别表明尿囊素(即 UA 氧化的最终产物)实际上并未参与协同机制,而是吸附在电极表面的氧化 UA 产物二亚胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号