当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into the Unique Ligation of [Fe4S4] Iron–Sulfur Clusters
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2018-03-26 , DOI: 10.1002/ejic.201800165 Adam Kubas 1 , Paweł Maszota 1
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2018-03-26 , DOI: 10.1002/ejic.201800165 Adam Kubas 1 , Paweł Maszota 1
Affiliation
|
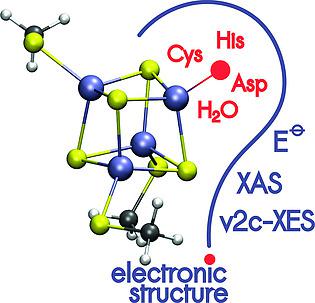
[Fe4S4] iron–sulfur clusters are typically anchored to the protein scaffold via four cysteine residues, but in a number of proteins one of the Cys is replaced with another ligand. The biological role of such a replacement is unknown although essential to maintain catalytic activity. Here, we explore the geometries and electronic structures of 3:1 site differentiated model compounds [Fe4S4][S(CH3)]3(L) using quantum chemical methods. The unique ligands L were chosen to represent biologically relevant molecules: methanethiol (represents cysteine), acetic acid (asparagine), 4‐methylimidazole (histidine), and water/hydroxyl anion. Each ligand was considered as deprotonated anion or protonated neutral molecule. We found the replacements do not influence structure of the cluster significantly. On the contrary, the impact on the reduction potentials is visible and may reach up to 0.2 V depending on the charge of the ligand. We also studied previously unconsidered asymmetry in excess charge distribution in a reduced [Fe4S4]+ cubane caused by a unique ligation. Our findings show that protonation/deprotonation reactions may provide gating mechanisms in the electron flow carried by iron–sulfur clusters. Additionally, we calculated X‐ray absorption (XAS) and emission (XES) spectra of all compounds under investigation. Particularly, theoretical XES show clear features characteristic to each unique ligand. However, their intensity may be too small to be observed experimentally.
中文翻译:

[Fe 4 S 4 ]铁-硫团簇唯一连接的理论见解
[Fe 4 S 4 ]铁-硫簇通常通过四个半胱氨酸残基锚定在蛋白质支架上,但是在许多蛋白质中,一个Cys被另一个配体取代。尽管对于维持催化活性至关重要,但这种替代物的生物学作用尚不清楚。在这里,我们探讨了3:1位置区分的模型化合物[Fe 4 S 4 ] [S(CH 3)] 3的几何形状和电子结构(l)采用量子化学方法。选择独特的配体L来代表生物学上相关的分子:甲硫醇(代表半胱氨酸),乙酸(天冬酰胺),4-甲基咪唑(组氨酸)和水/羟基阴离子。每个配体被认为是去质子化的阴离子或质子化的中性分子。我们发现替代品不会显着影响集群的结构。相反,对还原电势的影响是可见的,取决于配体的电荷,可能达到0.2V。我们还研究了还原[Fe 4 S 4 ] +中过量电荷分布中先前未考虑的不对称性古巴因独特的结扎而引起。我们的发现表明,质子化/去质子化反应可能在铁硫团簇携带的电子流中提供门控机制。此外,我们还计算了所有正在研究的化合物的X射线吸收(XAS)和发射(XES)光谱。特别地,理论XES显示出每种独特配体所特有的清晰特征。但是,它们的强度可能太小而无法通过实验观察到。
更新日期:2018-05-15
中文翻译:

[Fe 4 S 4 ]铁-硫团簇唯一连接的理论见解
[Fe 4 S 4 ]铁-硫簇通常通过四个半胱氨酸残基锚定在蛋白质支架上,但是在许多蛋白质中,一个Cys被另一个配体取代。尽管对于维持催化活性至关重要,但这种替代物的生物学作用尚不清楚。在这里,我们探讨了3:1位置区分的模型化合物[Fe 4 S 4 ] [S(CH 3)] 3的几何形状和电子结构(l)采用量子化学方法。选择独特的配体L来代表生物学上相关的分子:甲硫醇(代表半胱氨酸),乙酸(天冬酰胺),4-甲基咪唑(组氨酸)和水/羟基阴离子。每个配体被认为是去质子化的阴离子或质子化的中性分子。我们发现替代品不会显着影响集群的结构。相反,对还原电势的影响是可见的,取决于配体的电荷,可能达到0.2V。我们还研究了还原[Fe 4 S 4 ] +中过量电荷分布中先前未考虑的不对称性古巴因独特的结扎而引起。我们的发现表明,质子化/去质子化反应可能在铁硫团簇携带的电子流中提供门控机制。此外,我们还计算了所有正在研究的化合物的X射线吸收(XAS)和发射(XES)光谱。特别地,理论XES显示出每种独特配体所特有的清晰特征。但是,它们的强度可能太小而无法通过实验观察到。



























 京公网安备 11010802027423号
京公网安备 11010802027423号