当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
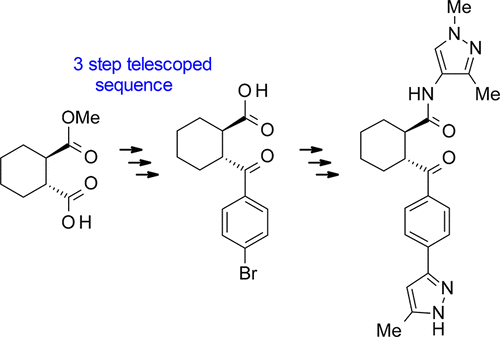
A Practical Telescoped Three-Step Sequence for the Preparation of (1R,2R)-2-(4-Bromobenzoyl)cyclohexanecarboxylic Acid: A Key Building Block Used in One of Our Drug Development Projects
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1021/acs.oprd.8b00066 Staffan Karlsson 1 , Rolf Bergman 2 , Johan Broddefalk 2 , Christian Löfberg 2 , Peter R. Moore 3 , Andrew Stark 3 , Hans Emtenäs 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1021/acs.oprd.8b00066 Staffan Karlsson 1 , Rolf Bergman 2 , Johan Broddefalk 2 , Christian Löfberg 2 , Peter R. Moore 3 , Andrew Stark 3 , Hans Emtenäs 1
Affiliation
|
A large-scale, robust telescoped process involving acid chloride generation and Friedel–Crafts acylation followed by hydrolysis of an ester was developed for the manufacture of a homochiral disubstituted cyclohexane. Chromatography was avoided, and instead, crystallization was employed to furnish the pure carboxylic acid. This acid was further used as a key building block for the synthesis of drug candidates via amide bond formation using various amines followed by a Suzuki coupling with a pyrazole pinacol ester. The total synthesis of one of the drug candidates on a multihundred gram scale is described.
中文翻译:

(1 R,2 R)-2-(4-溴苯甲酰基)环己烷羧酸制备的实用伸缩三步程序:在我们的药物开发项目之一中使用的关键构件
开发了一种大规模,鲁棒的伸缩过程,该过程涉及酰氯的产生和Friedel-Crafts的酰化作用,然后将其水解成酯,以生产均手性二取代的环己烷。避免了色谱法,取而代之的是通过结晶来提供纯的羧酸。该酸还被用作通过使用各种胺形成酰胺键,然后与吡唑频哪醇酯进行Suzuki偶联来合成候选药物的关键组成部分。描述了数百克规模的候选药物之一的总合成。
更新日期:2018-04-27
中文翻译:

(1 R,2 R)-2-(4-溴苯甲酰基)环己烷羧酸制备的实用伸缩三步程序:在我们的药物开发项目之一中使用的关键构件
开发了一种大规模,鲁棒的伸缩过程,该过程涉及酰氯的产生和Friedel-Crafts的酰化作用,然后将其水解成酯,以生产均手性二取代的环己烷。避免了色谱法,取而代之的是通过结晶来提供纯的羧酸。该酸还被用作通过使用各种胺形成酰胺键,然后与吡唑频哪醇酯进行Suzuki偶联来合成候选药物的关键组成部分。描述了数百克规模的候选药物之一的总合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号