当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
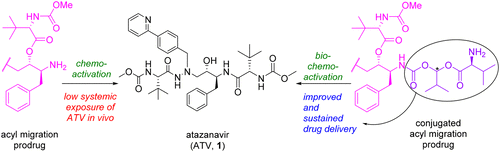
Coupling of an Acyl Migration Prodrug Strategy with Bio-activation To Improve Oral Delivery of the HIV-1 Protease Inhibitor Atazanavir
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-04-25 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00277 Murugaiah A. M. Subbaiah , Nicholas A. Meanwell , John F. Kadow , Lakshumanan Subramani , Mathiazhagan Annadurai , Thangeswaran Ramar , Salil D. Desai , Sarmistha Sinha , Murali Subramanian , Sandhya Mandlekar , Srikanth Sridhar , Shweta Padmanabhan , Priyadeep Bhutani , Rambabu Arla , Susan M. Jenkins , Mark R. Krystal , Chunfu Wang , Ramakanth Sarabu
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-04-25 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00277 Murugaiah A. M. Subbaiah , Nicholas A. Meanwell , John F. Kadow , Lakshumanan Subramani , Mathiazhagan Annadurai , Thangeswaran Ramar , Salil D. Desai , Sarmistha Sinha , Murali Subramanian , Sandhya Mandlekar , Srikanth Sridhar , Shweta Padmanabhan , Priyadeep Bhutani , Rambabu Arla , Susan M. Jenkins , Mark R. Krystal , Chunfu Wang , Ramakanth Sarabu
|
HIV-1 protease inhibitors (PIs), which include atazanavir (ATV, 1), remain important medicines to treat HIV-1 infection. However, they are characterized by poor oral bioavailability and a need for boosting with a pharmacokinetic enhancer, which results in additional drug–drug interactions that are sometimes difficult to manage. We investigated a chemo-activated, acyl migration-based prodrug design approach to improve the pharmacokinetic profile of 1 but failed to obtain improved oral bioavailability over dosing the parent drug in rats. This strategy was refined by conjugating the amine with a promoiety designed to undergo bio-activation, as a means of modulating the subsequent chemo-activation. This culminated in a lead prodrug that (1) yielded substantially better oral drug delivery of 1 when compared to the parent itself, the simple acyl migration-based prodrug, and the corresponding simple l-Val prodrug, (2) acted as a depot which resulted in a sustained release of the parent drug in vivo, and (3) offered the benefit of mitigating the pH-dependent absorption associated with 1, thereby potentially reducing the risk of decreased bioavailability with concurrent use of stomach-acid-reducing drugs.
中文翻译:

酰基转移前药策略与生物活化的耦合,以改善HIV-1蛋白酶抑制剂阿扎那韦的口服传递
HIV-1蛋白酶抑制剂(PIs)包括atazanavir(ATV,1)仍然是治疗HIV-1感染的重要药物。但是,它们的特点是口服生物利用度差,并且需要使用药代动力学增强剂进行增强,从而导致有时难以管理的其他药物相互作用。我们研究了一种基于化学活化,酰基转移的前药设计方法,以改善1的药代动力学。但与给大鼠服用母体药物相比,口服生物利用度没有提高。通过将胺与旨在进行生物活化的蛋白缀合来完善此策略,以作为调节后续化学活化的手段。这最终导致在铅前体药物:(1)产生的显着更好的口服药物递送1相对于亲本本身,简单的基于酰基迁移的前药,和相应的简单,当升-Val前药,(2)充当一个贮库,其导致母体药物在体内的持续释放,并且(3)具有减轻与1相关的pH依赖性吸收的好处,从而潜在地降低了同时使用降低胃酸的药物导致生物利用度降低的风险。
更新日期:2018-04-25
中文翻译:

酰基转移前药策略与生物活化的耦合,以改善HIV-1蛋白酶抑制剂阿扎那韦的口服传递
HIV-1蛋白酶抑制剂(PIs)包括atazanavir(ATV,1)仍然是治疗HIV-1感染的重要药物。但是,它们的特点是口服生物利用度差,并且需要使用药代动力学增强剂进行增强,从而导致有时难以管理的其他药物相互作用。我们研究了一种基于化学活化,酰基转移的前药设计方法,以改善1的药代动力学。但与给大鼠服用母体药物相比,口服生物利用度没有提高。通过将胺与旨在进行生物活化的蛋白缀合来完善此策略,以作为调节后续化学活化的手段。这最终导致在铅前体药物:(1)产生的显着更好的口服药物递送1相对于亲本本身,简单的基于酰基迁移的前药,和相应的简单,当升-Val前药,(2)充当一个贮库,其导致母体药物在体内的持续释放,并且(3)具有减轻与1相关的pH依赖性吸收的好处,从而潜在地降低了同时使用降低胃酸的药物导致生物利用度降低的风险。



























 京公网安备 11010802027423号
京公网安备 11010802027423号