当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Studies on the interactions of 5-R-3-(2-pyridyl)-1,2,4-triazines with arynes: inverse demand aza-Diels–Alder reaction versus aryne-mediated domino process†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1039/c8ob00847g Dmitry S. Kopchuk 1, 2, 3, 4, 5 , Igor L. Nikonov 1, 2, 3, 4, 5 , Albert F. Khasanov 1, 2, 3, 4, 5 , Kousik Giri 6, 7, 8, 9, 10 , Sougata Santra 1, 2, 3, 4, 5 , Igor S. Kovalev 1, 2, 3, 4, 5 , Emiliya V. Nosova 1, 2, 3, 4, 5 , Sravya Gundala 1, 2, 3, 4, 5 , Padmavathi Venkatapuram 10, 11, 12, 13 , Grigory V. Zyryanov 1, 2, 3, 4, 5 , Adinath Majee 10, 11, 14, 15 , Oleg N. Chupakhin 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1039/c8ob00847g Dmitry S. Kopchuk 1, 2, 3, 4, 5 , Igor L. Nikonov 1, 2, 3, 4, 5 , Albert F. Khasanov 1, 2, 3, 4, 5 , Kousik Giri 6, 7, 8, 9, 10 , Sougata Santra 1, 2, 3, 4, 5 , Igor S. Kovalev 1, 2, 3, 4, 5 , Emiliya V. Nosova 1, 2, 3, 4, 5 , Sravya Gundala 1, 2, 3, 4, 5 , Padmavathi Venkatapuram 10, 11, 12, 13 , Grigory V. Zyryanov 1, 2, 3, 4, 5 , Adinath Majee 10, 11, 14, 15 , Oleg N. Chupakhin 1, 2, 3, 4, 5
Affiliation
|
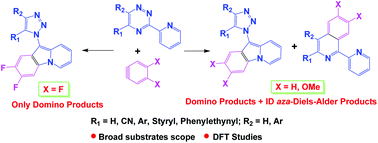
The interactions between substituted 5-R-3-(pyridyl-2)-1,2,4-triazines with in situ generated substituted aryne intermediates have been studied. The reaction afforded either inverse demand (ID) aza-Diels–Alder products or 1,2,4-triazine ring rearrangement (domino) products as major ones depending on the nature of both the substituents at the C5 position of the 1,2,4-triazine core or in the aryne moiety. The structures of the key products were confirmed based on X-ray data. Based on the density functional theoretical (DFT) studies of the Diels–Alder transition state geometries, the influence of the nature of arynes on the direction of the 1,2,4-triazine transformation has been proposed.
中文翻译:

5- R -3-(2-吡啶基)-1,2,4-三嗪与芳烃相互作用的研究:逆需求aza-Diels–Alder反应与芳烃介导的多米诺过程†
已经研究了取代的5 - R -3-(吡啶基-2)-1,2,4-三嗪与原位生成的取代的芳烃中间体之间的相互作用。根据1,2,5碳原子上两个取代基的性质,反应可提供反需求(ID)的aza-Diels-Alder产品或1,2,4-三嗪环重排(domino)产品作为主要产品。 4-三嗪核或在芳烃部分。根据X射线数据确定了关键产品的结构。基于Diels–Alder过渡态几何的密度泛函理论(DFT)研究,提出了芳烃性质对1,2,4-三嗪转化方向的影响。
更新日期:2018-04-24
中文翻译:

5- R -3-(2-吡啶基)-1,2,4-三嗪与芳烃相互作用的研究:逆需求aza-Diels–Alder反应与芳烃介导的多米诺过程†
已经研究了取代的5 - R -3-(吡啶基-2)-1,2,4-三嗪与原位生成的取代的芳烃中间体之间的相互作用。根据1,2,5碳原子上两个取代基的性质,反应可提供反需求(ID)的aza-Diels-Alder产品或1,2,4-三嗪环重排(domino)产品作为主要产品。 4-三嗪核或在芳烃部分。根据X射线数据确定了关键产品的结构。基于Diels–Alder过渡态几何的密度泛函理论(DFT)研究,提出了芳烃性质对1,2,4-三嗪转化方向的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号