当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-04-23 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01561 James R. Hill 1 , Avril A. B. Robertson 1, 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-04-23 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01561 James R. Hill 1 , Avril A. B. Robertson 1, 2
Affiliation
|
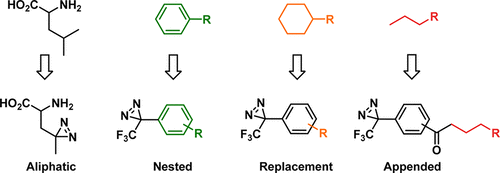
Target identification is a high-priority, albeit challenging, aspect of drug discovery. Diazirine-based photoaffinity probes (PAPs) can facilitate the process by covalently capturing transient molecular interactions. This can help identify target proteins and map the ligand’s interactome. Diazirine probes have even been incorporated by cellular machinery into proteins. Embarking on the synthesis of customized PAPs, containing either an aliphatic or trifluoromethyl phenyl diazirine, can be a considerable endeavor, particularly for medicinal chemists and chemical biologists new to the field. This review takes a synthetic focus, aiming to summarize available routes, propose new avenues, and illuminate recent advances in diazirine synthesis. Select examples of diazirine photoaffinity labeling applications have been included throughout to provide instructive definition of the advantages and limitations of the technology while simultaneously highlighting how these reagents can be applied in a practical sense.
中文翻译:

捕捞药物目标:专注于二嗪基光亲和探针合成
目标识别是药物发现的高度优先事项,尽管具有挑战性。基于重氮嗪的光亲和探针(PAP)可通过共价捕获瞬态分子相互作用来促进该过程。这可以帮助鉴定靶蛋白并定位配体的相互作用组。二嗪嗪探针甚至已经通过细胞机器掺入了蛋白质中。着手合成包含脂肪族或三氟甲基苯基二叠氮基的定制PAP可能是一项相当大的努力,特别是对于本领域刚接触的药物化学家和化学生物学家而言。这篇综述着眼于综合,旨在总结可用的路线,提出新的途径,并阐明二嗪合成的最新进展。
更新日期:2018-04-23
中文翻译:

捕捞药物目标:专注于二嗪基光亲和探针合成
目标识别是药物发现的高度优先事项,尽管具有挑战性。基于重氮嗪的光亲和探针(PAP)可通过共价捕获瞬态分子相互作用来促进该过程。这可以帮助鉴定靶蛋白并定位配体的相互作用组。二嗪嗪探针甚至已经通过细胞机器掺入了蛋白质中。着手合成包含脂肪族或三氟甲基苯基二叠氮基的定制PAP可能是一项相当大的努力,特别是对于本领域刚接触的药物化学家和化学生物学家而言。这篇综述着眼于综合,旨在总结可用的路线,提出新的途径,并阐明二嗪合成的最新进展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号