当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bioinspired Oxidative Cyclization of the Geissoschizine Skeleton for the Total Synthesis of (−)‐17‐nor‐Excelsinidine
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-23 , DOI: 10.1002/anie.201802610 Maxime Jarret 1 , Aurélien Tap 1 , Cyrille Kouklovsky 1 , Erwan Poupon 2 , Laurent Evanno 2 , Guillaume Vincent 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-23 , DOI: 10.1002/anie.201802610 Maxime Jarret 1 , Aurélien Tap 1 , Cyrille Kouklovsky 1 , Erwan Poupon 2 , Laurent Evanno 2 , Guillaume Vincent 1
Affiliation
|
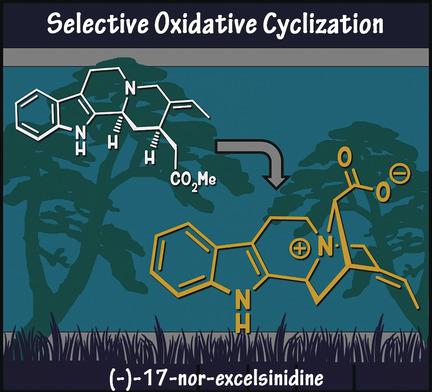
We report the first total synthesis of (−)‐17‐nor‐excelsinidine, a zwitterionic monoterpene indole alkaloid that displays an unusual N4−C16 connection. Inspired by the postulated biosynthesis, we explored an oxidative coupling approach from the geissoschizine framework to forge the key ammonium–acetate connection. Two strategies allowed us to achieve this goal, namely an intramolecular nucleophilic substitution on a 16‐chlorolactam with the N4 nitrogen atom or a direct I2‐mediated N4−C16 oxidative coupling from the enolate of geissoschizine.
中文翻译:

生物启发性的Geissoschizine骨架的氧化环化反应,可合成(-)-17-nor-Excelsinidine
我们报告了(−)-17-nor-excelsinidine的第一个全合成,这是一种两性离子单萜吲哚生物碱,显示出异常的N4-C16连接。受假定的生物合成的启发,我们探索了Geissoschizine骨架的氧化偶联方法,以建立关键的醋酸铵连接。有两种策略使我们得以实现这一目标,即在16-氯内酰胺上用N4氮原子进行分子内亲核取代或从Geissoschizine的烯醇化物中直接进行I 2介导的N4-C16氧化偶联。
更新日期:2018-04-23
中文翻译:

生物启发性的Geissoschizine骨架的氧化环化反应,可合成(-)-17-nor-Excelsinidine
我们报告了(−)-17-nor-excelsinidine的第一个全合成,这是一种两性离子单萜吲哚生物碱,显示出异常的N4-C16连接。受假定的生物合成的启发,我们探索了Geissoschizine骨架的氧化偶联方法,以建立关键的醋酸铵连接。有两种策略使我们得以实现这一目标,即在16-氯内酰胺上用N4氮原子进行分子内亲核取代或从Geissoschizine的烯醇化物中直接进行I 2介导的N4-C16氧化偶联。


























 京公网安备 11010802027423号
京公网安备 11010802027423号