当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrodimerization of N‐Alkoxyamides for Zinc(II) Catalyzed Phenolic Ester Synthesis under Mild Reaction Conditions
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-05-08 , DOI: 10.1002/adsc.201701646 Kripa Subramanian 1 , Subhash L. Yedage 1 , Bhalchandra M. Bhanage 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-05-08 , DOI: 10.1002/adsc.201701646 Kripa Subramanian 1 , Subhash L. Yedage 1 , Bhalchandra M. Bhanage 1
Affiliation
|
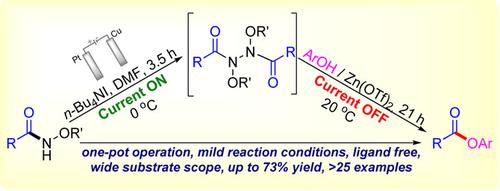
An electrochemical On‐Off method for phenolic ester synthesis from N‐alkoxyamides has been reported. This one‐pot protocol begins with rapid and selective electrodimerization of the amide using n‐Bu4NI (TBAI) as an electrocatalyst. The reaction proceeds further in the absence of current via Zn catalyzed C−N bond activation of the amide dimer followed by its coupling with phenol to form the ester. The present methodology is ligand‐free and takes place under mild reaction conditions. This transformation incorporates a wide variety of phenols and amide substrates leading to the formation of functionalized esters highlighting its versatility.
中文翻译:

轻度反应条件下N-烷氧基酰胺的电二聚反应,用于锌(II)催化的酚酯合成
已经报道了从N-烷氧基酰胺合成酚酯的电化学开-关方法。这种一锅法的协议开始于使用n- Bu 4 NI(TBAI)作为电催化剂对酰胺进行快速和选择性的电二聚。在不存在电流的情况下,该反应会进一步通过酰胺催化的二聚体的Zn催化C-N键活化而进行,然后将其与苯酚偶联形成酯。本方法学不含配体,在温和的反应条件下进行。这种转变结合了多种酚和酰胺底物,导致形成功能化的酯,从而突出了其多功能性。
更新日期:2018-05-08
中文翻译:

轻度反应条件下N-烷氧基酰胺的电二聚反应,用于锌(II)催化的酚酯合成
已经报道了从N-烷氧基酰胺合成酚酯的电化学开-关方法。这种一锅法的协议开始于使用n- Bu 4 NI(TBAI)作为电催化剂对酰胺进行快速和选择性的电二聚。在不存在电流的情况下,该反应会进一步通过酰胺催化的二聚体的Zn催化C-N键活化而进行,然后将其与苯酚偶联形成酯。本方法学不含配体,在温和的反应条件下进行。这种转变结合了多种酚和酰胺底物,导致形成功能化的酯,从而突出了其多功能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号