Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biochemical and kinetic characterization of laccase and manganese peroxidase from novel Klebsiella pneumoniae strains and their application in bioethanol production
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8ra01204k Nisha Gaur 1 , Korrapati Narasimhulu 1 , Pydisetty Y 2
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8ra01204k Nisha Gaur 1 , Korrapati Narasimhulu 1 , Pydisetty Y 2
Affiliation
|
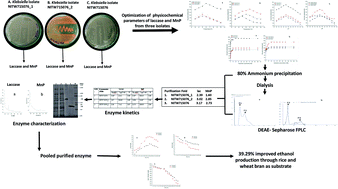
Laccase (lac) and manganese peroxidase (MnP) enzymes from the novel Klebsiella pneumoniae isolates, grown on lignin basic media (LBM) were purified by 80% ammonium sulphate fractionation, dialysis and DEAE-sepharose column chromatography. The optimum temperatures for laccase production were 60 °C, 50 °C and 50 °C and for MnP production were 50 °C, 70 °C and 60 °C from NITW715076_2, NITW715076_1 and NITW715076 isolates, respectively. The optimal pH for production was found to be 5 for production of both the enzymes from all the isolates. 2.8–3.5 fold enzyme purification was achieved retaining around 60–70% of the initial activity. SDS-PAGE revealed the molecular mass of laccase and MnP to be 66 kDa and 48 kDa, respectively. The substrate ABTS and MnSO4 exhibited more specificity towards NITW715075_2 derived laccase and MnP (lac: Km = 0.38 mM, Vmax = 71.42 U ml−1; MnP: Km = 0.17 mM, Vmax = 106.38 U ml−1) compared to NITW715076_1 (lac: Km = 3.97 mM, Vmax = 148.8 U ml−1; MnP: Km = 0.90 mM, Vmax = 114.67 U ml−1) and NITW715076 (lac: Km = 0.46 mM, Vmax = 23.42 U ml−1; MnP: Km = 0.19 mM, Vmax = 108.10 U ml−1) derived. L-Cysteine and sodium azide imposed a strong inhibitory effect on the activities of both the enzymes. EDTA inhibited laccase and MnP activity at higher concentration. SDS strongly inhibited activity while for MnP it showed less inhibitory effect. The enzymes were employed for ethanol production from rice and wheat bran biomass which showed 39.29% improved production compared to control. After evaluating the applicability of these enzymes it can be suggested that the ligninolytic enzyme of Klebsiella pneumoniae isolates could be effectively employed in enhanced ethanol production and could be explored for other putative applications.
中文翻译:

新型肺炎克雷伯菌漆酶和锰过氧化物酶的生化和动力学特征及其在生物乙醇生产中的应用
来自新型肺炎克雷伯菌分离株的漆酶 (lac) 和锰过氧化物酶 (MnP) 酶通过 80% 硫酸铵分级分离、透析和 DEAE-琼脂糖柱层析纯化,在木质素基础培养基 (LBM) 上生长。NITW715076_2、NITW715076_1 和 NITW715076 分离株的漆酶生产的最佳温度分别为 60 °C、50 °C 和 50 °C,MnP 生产的最佳温度分别为 50 °C、70 °C 和 60 °C。发现从所有分离物中生产两种酶的最佳生产 pH 值为 5。实现了 2.8-3.5 倍的酶纯化,保留了大约 60-70% 的初始活性。SDS-PAGE 显示漆酶和 MnP 的分子量分别为 66 kDa 和 48 kDa。底物 ABTS 和 MnSO 4与 NITW715076_1 (lac: K m = 0.38 mM, V max = 71.42 U ml -1 ; MnP: K m = 0.17 mM, V max = 106.38 U ml -1 ) 相比,对 NITW715075_2 衍生的漆酶和 MnP 表现出更高的特异性(lac: K m = 3.97 mM,V max = 148.8 U ml -1;MnP:K m = 0.90 mM,V max = 114.67 U ml -1)和 NITW715076(lac:K m = 0.46 mM,V max = 23.42 U ml - 1 ; 锰磷:K m = 0.19 mM,V max = 108.10 U ml -1 ) 导出。L-半胱氨酸和叠氮化钠对这两种酶的活性都有很强的抑制作用。EDTA 在较高浓度下抑制漆酶和 MnP 活性。SDS 强烈抑制活性,而对 MnP 显示出较少的抑制作用。这些酶被用于从稻米和麦麸生物质中生产乙醇,与对照相比,产量提高了 39.29%。在评估了这些酶的适用性之后,可以建议肺炎克雷伯菌分离物的木质素分解酶可以有效地用于增强乙醇生产,并且可以探索其他推定的应用。
更新日期:2018-04-20
中文翻译:

新型肺炎克雷伯菌漆酶和锰过氧化物酶的生化和动力学特征及其在生物乙醇生产中的应用
来自新型肺炎克雷伯菌分离株的漆酶 (lac) 和锰过氧化物酶 (MnP) 酶通过 80% 硫酸铵分级分离、透析和 DEAE-琼脂糖柱层析纯化,在木质素基础培养基 (LBM) 上生长。NITW715076_2、NITW715076_1 和 NITW715076 分离株的漆酶生产的最佳温度分别为 60 °C、50 °C 和 50 °C,MnP 生产的最佳温度分别为 50 °C、70 °C 和 60 °C。发现从所有分离物中生产两种酶的最佳生产 pH 值为 5。实现了 2.8-3.5 倍的酶纯化,保留了大约 60-70% 的初始活性。SDS-PAGE 显示漆酶和 MnP 的分子量分别为 66 kDa 和 48 kDa。底物 ABTS 和 MnSO 4与 NITW715076_1 (lac: K m = 0.38 mM, V max = 71.42 U ml -1 ; MnP: K m = 0.17 mM, V max = 106.38 U ml -1 ) 相比,对 NITW715075_2 衍生的漆酶和 MnP 表现出更高的特异性(lac: K m = 3.97 mM,V max = 148.8 U ml -1;MnP:K m = 0.90 mM,V max = 114.67 U ml -1)和 NITW715076(lac:K m = 0.46 mM,V max = 23.42 U ml - 1 ; 锰磷:K m = 0.19 mM,V max = 108.10 U ml -1 ) 导出。L-半胱氨酸和叠氮化钠对这两种酶的活性都有很强的抑制作用。EDTA 在较高浓度下抑制漆酶和 MnP 活性。SDS 强烈抑制活性,而对 MnP 显示出较少的抑制作用。这些酶被用于从稻米和麦麸生物质中生产乙醇,与对照相比,产量提高了 39.29%。在评估了这些酶的适用性之后,可以建议肺炎克雷伯菌分离物的木质素分解酶可以有效地用于增强乙醇生产,并且可以探索其他推定的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号