当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of Selective, Cell Active Inhibitors of Protein Arginine Methyltransferase 5 through Structure-Based Virtual Screening and Biological Assays
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jcim.8b00050 Fei Ye 1 , Weiyao Zhang 1 , Xiaoqing Ye 1 , Jia Jin 1 , Zhengbing Lv 1 , Cheng Luo 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jcim.8b00050 Fei Ye 1 , Weiyao Zhang 1 , Xiaoqing Ye 1 , Jia Jin 1 , Zhengbing Lv 1 , Cheng Luo 2
Affiliation
|
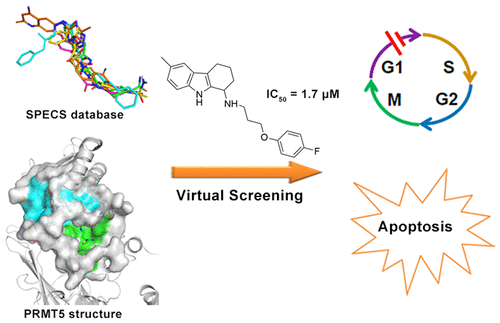
Protein arginine methyltransferase 5 (PRMT5), a type II PRMT enzyme, is reported as an important therapeutic target in leukemia and lymphoma. In the present study, based on the combination of virtual screening and biochemical validations, we discovered a series of small-molecule inhibitors targeting PRMT5. Among those, DC_Y134 exhibited the most potent activity with IC50 value of 1.7 μM and displayed good selectivity against other methyltransferases. Further treatment with DC_Y134 inhibited the proliferation of several hematological malignancy cell lines by causing cell cycle arrest and apoptosis. Western blot assays indicated that DC_Y134 reduced the cellular symmetrically dimethylated levels. In addition, we analyzed the binding mode of DC_Y134 through molecular docking, which revealed that DC_Y134 occupies the binding site of substrate arginine and explained the selectivity of this inhibitor. Taken together, compound DC_Y134 could be used to elucidate the biological roles of PRMT5 and serve as a lead compound for treatment of hematologic malignancies.
中文翻译:

通过基于结构的虚拟筛选和生物学分析鉴定蛋白精氨酸甲基转移酶5的选择性细胞活性抑制剂。
蛋白质精氨酸甲基转移酶5(PRMT5)是II型PRMT酶,据报道是白血病和淋巴瘤的重要治疗靶标。在本研究中,基于虚拟筛选和生化验证的结合,我们发现了一系列针对PRMT5的小分子抑制剂。其中,DC_Y134表现出最强的IC 50活性值为1.7μM,对其他甲基转移酶表现出良好的选择性。DC_Y134的进一步处理通过引起细胞周期停滞和凋亡而抑制了几种血液系统恶性细胞系的增殖。Western印迹分析表明DC_Y134降低了细胞对称的二甲基化水平。此外,我们通过分子对接分析了DC_Y134的结合方式,发现DC_Y134占据了精氨酸底物的结合位点,并解释了该抑制剂的选择性。综上所述,化合物DC_Y134可用于阐明PRMT5的生物学作用,并作为治疗血液系统恶性肿瘤的先导化合物。
更新日期:2018-04-19
中文翻译:

通过基于结构的虚拟筛选和生物学分析鉴定蛋白精氨酸甲基转移酶5的选择性细胞活性抑制剂。
蛋白质精氨酸甲基转移酶5(PRMT5)是II型PRMT酶,据报道是白血病和淋巴瘤的重要治疗靶标。在本研究中,基于虚拟筛选和生化验证的结合,我们发现了一系列针对PRMT5的小分子抑制剂。其中,DC_Y134表现出最强的IC 50活性值为1.7μM,对其他甲基转移酶表现出良好的选择性。DC_Y134的进一步处理通过引起细胞周期停滞和凋亡而抑制了几种血液系统恶性细胞系的增殖。Western印迹分析表明DC_Y134降低了细胞对称的二甲基化水平。此外,我们通过分子对接分析了DC_Y134的结合方式,发现DC_Y134占据了精氨酸底物的结合位点,并解释了该抑制剂的选择性。综上所述,化合物DC_Y134可用于阐明PRMT5的生物学作用,并作为治疗血液系统恶性肿瘤的先导化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号