当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational and Experimental Investigations of the Role of Water and Alcohols in the Desorption of Heterocyclic Aromatic Compounds from Kaolinite in Toluene
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b12655 Mateus R. Lage 1, 2, 3, 4 , Gustave K. Dedzo 5, 6, 7 , Stanislav R. Stoyanov 1, 2, 8, 9 , WenJuan Huang 1, 2 , Sergey Gusarov 1, 2 , José Walkimar de M. Carneiro 4 , Christian Detellier 5, 6 , Andriy Kovalenko 1, 2
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b12655 Mateus R. Lage 1, 2, 3, 4 , Gustave K. Dedzo 5, 6, 7 , Stanislav R. Stoyanov 1, 2, 8, 9 , WenJuan Huang 1, 2 , Sergey Gusarov 1, 2 , José Walkimar de M. Carneiro 4 , Christian Detellier 5, 6 , Andriy Kovalenko 1, 2
Affiliation
|
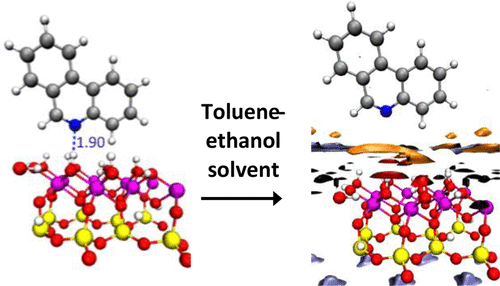
Nonaqueous extraction is an attractive alternative to the currently employed warm water process for extraction of bitumen from oil sands, as it could use less energy and water. Hydroxylated cosolvents, such as alcohols, that compete for the adsorptive clay surfaces and help release bitumen components could help improve bitumen recovery. The water naturally present in oil sand also affects oil–mineral interactions. Electronic structure methods and the statistical-mechanical 3D-RISM-KH molecular theory of solvation as well as experimental desorption measurements are employed to study the effects of water and aliphatic alcohol cosolvents in toluene solvent on the desorption of fused pyridinic heterocycles (ArN) from kaolinite. The geometries of phenanthridine and acridine (representative of pyridinic heterocycles of petroleum asphaltenes) adsorbed on the kaolinite clay surface are optimized in periodic boundary conditions using density functional theory. The 3D-RISM-KH method is employed to calculate the solvation free energy and potential of mean force for adsorption of the heterocycles on kaolinite in pure and alcohol-containing toluene. The potentials of mean force show that the adsorption of the fused pyridines on kaolinite is stronger in pure toluene than in toluene mixed with aliphatic alcohol. Analysis of the mechanism of desorption of phenanthridine and acridine from kaolinite in toluene containing alcohol reveals that the alcohol stabilizes both the pyridinic moiety and kaolinite platelet by hydrogen bonding, thus disrupting the ArN···HO–Al(kaolinite) hydrogen bond. A mechanism for retention of toluene on kaolinite is also highlighted. Experimental studies of the desorption of fused pyridines from an ArN–kaolinite aggregate show that in water-saturated toluene the rate of desorption of the phenanthridine from kaolinite is twice as high as that in dry toluene. The experimental and computational results show that water and aliphatic alcohols in toluene help desorb pyridinic heterocycles from kaolinite, a clay mineral abundant in the oil sands. The presented insights are valuable for understanding the molecule-clay interactions in solution and relevant to improving the nonaqueous extraction of bitumen from oil sand.
中文翻译:

水和醇在高岭石中甲苯解吸杂环芳族化合物中的作用的计算和实验研究
非水萃取是目前使用的温水工艺从油砂中萃取沥青的一种有吸引力的替代方法,因为它可以使用较少的能量和水。竞争性的黏土表面并帮助释放沥青组分的羟基化助溶剂(如醇)可以帮助改善沥青的回收率。油砂中自然存在的水也会影响油矿相互作用。利用电子结构方法和统计力学的3D-RISM-KH溶剂化分子理论以及解吸实验,研究了甲苯溶剂中水和脂族醇助溶剂对熔融吡啶环杂环(ArN)从高岭石解吸的影响。使用密度泛函理论在周期性边界条件下优化了吸附在高岭石粘土表面上的菲啶和a啶的几何形状(代表石油沥青质的吡啶杂环)。使用3D-RISM-KH方法计算纯净和含酒精的甲苯中高岭石上杂环吸附的溶剂化自由能和平均力势。平均力的潜力表明,在纯甲苯中,熔融吡啶在高岭石上的吸附要强于与脂肪醇混合的甲苯。分析菲在含甲苯的甲苯中从高岭石中解吸出菲啶和a啶的机理表明,该醇通过氢键稳定吡啶基部分和高岭土血小板,因此破坏了ArN··HO-Al(高岭石)氢键。还强调了在高岭石上保留甲苯的机理。从ArN-高岭石聚集体中解吸吡啶的实验研究表明,在水饱和的甲苯中,菲啶从高岭石中的解吸速率是干甲苯中的两倍。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。从ArN-高岭石聚集体中解吸吡啶的实验研究表明,在水饱和的甲苯中,菲啶从高岭石中的解吸速率是干甲苯中的两倍。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。从ArN-高岭石聚集体中解吸吡啶的实验研究表明,在水饱和的甲苯中,菲啶从高岭石中的解吸速率是干甲苯中的两倍。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。
更新日期:2018-04-19
中文翻译:

水和醇在高岭石中甲苯解吸杂环芳族化合物中的作用的计算和实验研究
非水萃取是目前使用的温水工艺从油砂中萃取沥青的一种有吸引力的替代方法,因为它可以使用较少的能量和水。竞争性的黏土表面并帮助释放沥青组分的羟基化助溶剂(如醇)可以帮助改善沥青的回收率。油砂中自然存在的水也会影响油矿相互作用。利用电子结构方法和统计力学的3D-RISM-KH溶剂化分子理论以及解吸实验,研究了甲苯溶剂中水和脂族醇助溶剂对熔融吡啶环杂环(ArN)从高岭石解吸的影响。使用密度泛函理论在周期性边界条件下优化了吸附在高岭石粘土表面上的菲啶和a啶的几何形状(代表石油沥青质的吡啶杂环)。使用3D-RISM-KH方法计算纯净和含酒精的甲苯中高岭石上杂环吸附的溶剂化自由能和平均力势。平均力的潜力表明,在纯甲苯中,熔融吡啶在高岭石上的吸附要强于与脂肪醇混合的甲苯。分析菲在含甲苯的甲苯中从高岭石中解吸出菲啶和a啶的机理表明,该醇通过氢键稳定吡啶基部分和高岭土血小板,因此破坏了ArN··HO-Al(高岭石)氢键。还强调了在高岭石上保留甲苯的机理。从ArN-高岭石聚集体中解吸吡啶的实验研究表明,在水饱和的甲苯中,菲啶从高岭石中的解吸速率是干甲苯中的两倍。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。从ArN-高岭石聚集体中解吸吡啶的实验研究表明,在水饱和的甲苯中,菲啶从高岭石中的解吸速率是干甲苯中的两倍。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。从ArN-高岭石聚集体中解吸吡啶的实验研究表明,在水饱和的甲苯中,菲啶从高岭石中的解吸速率是干甲苯中的两倍。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。实验和计算结果表明,甲苯中的水和脂肪族醇有助于从高岭石(一种富含油砂的粘土矿物)中解吸吡啶系杂环。提出的见解对于理解溶液中的分子-粘土相互作用具有重要意义,并且与改善从油砂中非水萃取沥青有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号