当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Au Dendrite Electrocatalysts for CO2 Electrolysis
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.8b01831 Nathan T. Nesbitt 1 , Ming Ma 2 , Bartek J. Trześniewski 2 , Samantha Jaszewski 1 , Fazel Tafti 1 , Michael J. Burns 1 , Wilson A. Smith 2 , Michael J. Naughton 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.8b01831 Nathan T. Nesbitt 1 , Ming Ma 2 , Bartek J. Trześniewski 2 , Samantha Jaszewski 1 , Fazel Tafti 1 , Michael J. Burns 1 , Wilson A. Smith 2 , Michael J. Naughton 1
Affiliation
|
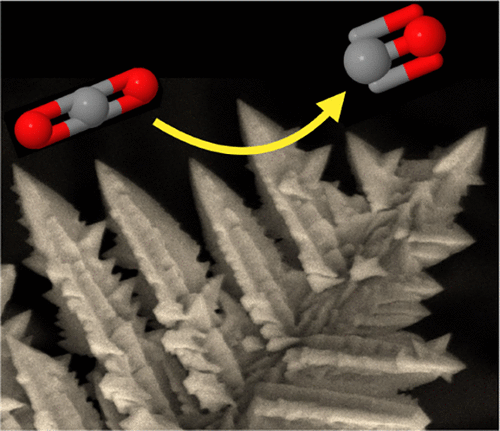
Electrochemical CO2 reduction can convert CO2 into fuels and valuable chemicals using renewable electricity, which provides a prospective path toward large-scale energy storage. Au nanostructured electrodes have demonstrated the best catalytic performance for CO2 conversion: high catalytic selectivity for CO formation at low overpotentials, high current density, and long-term durability. Here, we report selective electrocatalytic CO2 reduction to CO on nanostructured Au with various morphologies, prepared via electrocrystallization with a megahertz potential oscillation. X-ray diffraction showed that the proportion of {100} and {110} to {111} surfaces increased at more negative deposition potentials. Cyclic voltammetry showed the potential of zero charge on an Au film was approximately 0.35 V vs standard hydrogen electrode (SHE) and that the surface energy decreased by ∼1 eV/nm2 at −0.5 V vs SHE, tending to 0 within several volts in either direction. Scanning electron micrograms showed that the Au crystals grow primarily in the ⟨110⟩ directions. From these data, a model for crystallization from melts was adapted to calculate the roughening temperature of the {111}, {100}, and {110} Miller indices as 7000, 4000, and 1000 K, decreasing for more negative deposition potentials. This offers a framework for exposed facet control in electrocrystallization. In CO2 electrocatalysis, −0.35 V vs reversible hydrogen electrode was observed to be a turn-on potential for improved CO2 reduction activity; dendrites showed 50% Faradaic efficiency for CO production at more cathodic potentials. The Tafel slope was measured to be 40 mV/decade for {100} and {110}-rich Au dendrites and 110 mV/decade for {111}-dominated Au plates, suggesting the higher surface energy crystal facets may stabilize all of the CO2 reduction reaction intermediates.
中文翻译:

用于CO 2电解的金枝晶电催化剂
电化学还原CO 2可以使用可再生电力将CO 2转化为燃料和有价值的化学物质,这为大规模储能提供了前途。Au纳米结构电极已证明具有最佳的CO 2转化催化性能:在低过电势,高电流密度和长期耐用性下,对CO形成具有高催化选择性。在这里,我们报道了选择性电催化CO 2通过具有兆赫兹电势振荡的电结晶制备的具有各种形态的纳米结构的Au还原成CO。X射线衍射表明{100}和{110}对{111}表面的比例在更多的负沉积电势下增加。循环伏安法表明,与标准氢电极(SHE)相比,Au膜上的零电荷电势约为0.35 V,表面能降低了约1 eV / nm 2。在-0.5 V相对于SHE的情况下,在任一方向的几伏内趋于0。扫描电子显微图显示,Au晶体主要在⟨110⟩方向上生长。根据这些数据,从熔体中结晶的模型适用于计算{111},{100}和{110} Miller指数的粗化温度为7000、4000和1000 K,以降低负的沉积电位。这为电结晶中裸露面的控制提供了框架。在CO 2电催化中,相对于可逆氢电极,-0.35 V被观察到是改善CO 2的开启电势减少活动;树枝状晶体在更多的阴极电位下显示出50%的法拉第生产CO效率。对于{100}和{110}富金的枝晶,Tafel斜率测量为40 mV /十倍,对于{111}为主的Au板,Tafel斜率测得为110 mV /十倍,表明较高的表面能晶体刻面可以稳定所有CO。2还原反应中间体。
更新日期:2018-04-19
中文翻译:

用于CO 2电解的金枝晶电催化剂
电化学还原CO 2可以使用可再生电力将CO 2转化为燃料和有价值的化学物质,这为大规模储能提供了前途。Au纳米结构电极已证明具有最佳的CO 2转化催化性能:在低过电势,高电流密度和长期耐用性下,对CO形成具有高催化选择性。在这里,我们报道了选择性电催化CO 2通过具有兆赫兹电势振荡的电结晶制备的具有各种形态的纳米结构的Au还原成CO。X射线衍射表明{100}和{110}对{111}表面的比例在更多的负沉积电势下增加。循环伏安法表明,与标准氢电极(SHE)相比,Au膜上的零电荷电势约为0.35 V,表面能降低了约1 eV / nm 2。在-0.5 V相对于SHE的情况下,在任一方向的几伏内趋于0。扫描电子显微图显示,Au晶体主要在⟨110⟩方向上生长。根据这些数据,从熔体中结晶的模型适用于计算{111},{100}和{110} Miller指数的粗化温度为7000、4000和1000 K,以降低负的沉积电位。这为电结晶中裸露面的控制提供了框架。在CO 2电催化中,相对于可逆氢电极,-0.35 V被观察到是改善CO 2的开启电势减少活动;树枝状晶体在更多的阴极电位下显示出50%的法拉第生产CO效率。对于{100}和{110}富金的枝晶,Tafel斜率测量为40 mV /十倍,对于{111}为主的Au板,Tafel斜率测得为110 mV /十倍,表明较高的表面能晶体刻面可以稳定所有CO。2还原反应中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号