当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Water Adsorption and Reactions on α-Al2O3(0001) and α-Alumina Particles
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.8b01969 Nikolay G. Petrik 1 , Patricia L. Huestis 2 , Jay A. LaVerne 2 , Alexandr B. Aleksandrov 3 , Thomas M. Orlando 3 , Greg A. Kimmel 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.8b01969 Nikolay G. Petrik 1 , Patricia L. Huestis 2 , Jay A. LaVerne 2 , Alexandr B. Aleksandrov 3 , Thomas M. Orlando 3 , Greg A. Kimmel 1
Affiliation
|
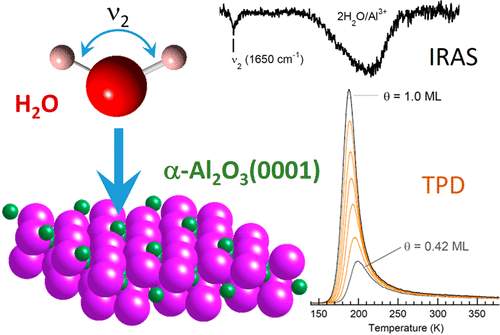
The adsorption and reaction of water on single crystal α-Al2O3(0001) in ultrahigh vacuum, and α-alumina particles in ambient conditions, were investigated using temperature-programmed desorption (TPD), infrared reflection absorption spectroscopy (IRAS), diffuse reflectance infrared Fourier transform spectra (DRIFTS), and other surface science techniques. For a water coverage of 1 H2O/(surface Al3+) on α-Al2O3(0001), no evidence for the surface hydroxyls expected from dissociative adsorption was observed in the infrared spectra, while the ν2 vibration of molecular water was observed. Electron-stimulated desorption of molecular water at low coverages also indicated molecular or mixed (molecular plus dissociative) adsorption. Analysis of Kr TPD spectra showed that the water films wet the alumina substrate and suggested that the films were initially growing layer-by-layer. In contrast with the single crystal results, DRIFTS of water adsorption on alumina particles indicated the presence of surface hydroxyls that persist even after annealing to high temperatures in oxygen. For water on α-Al2O3(0001) at coverages less than 0.3 H2O/(surface Al3+), water desorbed over a broad temperature range extending from ∼250 to 700 K. For larger coverages, water desorption occurred at temperatures between ∼160 and 250 K, consistent with desorption of molecular water. The results, which are consistent with at most a small amount of water dissociation on the Al-terminated (0001) surface, are difficult to reconcile with calculations suggesting that the barrier to dissociation is small. However, the results are consistent with recent vibrational sum frequency experiments showing that the hydroxylation of the Al-terminated (0001) surface takes many days even at ambient pressures and temperatures.
中文翻译:

水分子上的吸附和反应的α-Al 2 ö 3(0001)和α氧化铝粒子
在吸附和水反应单晶的α-Al 2 ö 3使用程序升温脱附(TPD),红外反射吸收光谱(IRAS)(0001)在超高真空,和α氧化铝在环境条件下的颗粒,进行了调查,漫反射红外傅立叶变换光谱(DRIFTS)和其他表面科学技术。为的1 H一水覆盖2 O /(表面的Al 3+)上的α-Al 2 ö 3(0001),用于从解离吸附预计在红外光谱中观察到的表面羟基没有证据,而ν 2观察到分子水的振动。电子刺激的低覆盖率分子水的脱附也表明分子或混合(分子加解离)的吸附。Kr TPD光谱分析表明,水膜润湿了氧化铝基材,表明该膜最初是逐层生长的。与单晶结果相反,氧化铝颗粒上水吸附的DRIFTS表明即使在氧气中退火至高温后,表面羟基仍存在。有关水的α-Al 2 ö 3(0001)在覆盖范围小于0.3ħ 2 O /(表面的Al 3+),水在约250至700 K的宽温度范围内解吸。对于较大的覆盖范围,水的解吸发生在约160至250 K的温度下,这与分子水的解吸一致。该结果与铝封端的(0001)表面上至多少量的水离解相符,难以与计算相符,这表明离解的障碍很小。但是,该结果与最近的振动总频率实验一致,该实验表明,即使在环境压力和温度下,铝终止(0001)表面的羟基化也要花费很多时间。
更新日期:2018-04-19
中文翻译:

水分子上的吸附和反应的α-Al 2 ö 3(0001)和α氧化铝粒子
在吸附和水反应单晶的α-Al 2 ö 3使用程序升温脱附(TPD),红外反射吸收光谱(IRAS)(0001)在超高真空,和α氧化铝在环境条件下的颗粒,进行了调查,漫反射红外傅立叶变换光谱(DRIFTS)和其他表面科学技术。为的1 H一水覆盖2 O /(表面的Al 3+)上的α-Al 2 ö 3(0001),用于从解离吸附预计在红外光谱中观察到的表面羟基没有证据,而ν 2观察到分子水的振动。电子刺激的低覆盖率分子水的脱附也表明分子或混合(分子加解离)的吸附。Kr TPD光谱分析表明,水膜润湿了氧化铝基材,表明该膜最初是逐层生长的。与单晶结果相反,氧化铝颗粒上水吸附的DRIFTS表明即使在氧气中退火至高温后,表面羟基仍存在。有关水的α-Al 2 ö 3(0001)在覆盖范围小于0.3ħ 2 O /(表面的Al 3+),水在约250至700 K的宽温度范围内解吸。对于较大的覆盖范围,水的解吸发生在约160至250 K的温度下,这与分子水的解吸一致。该结果与铝封端的(0001)表面上至多少量的水离解相符,难以与计算相符,这表明离解的障碍很小。但是,该结果与最近的振动总频率实验一致,该实验表明,即使在环境压力和温度下,铝终止(0001)表面的羟基化也要花费很多时间。


























 京公网安备 11010802027423号
京公网安备 11010802027423号