当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acetylenes in the Superbase-Promoted Assembly of Carbocycles and Heterocycles
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1021/acs.accounts.7b00618 Boris A. Trofimov 1 , Elena Yu. Schmidt 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1021/acs.accounts.7b00618 Boris A. Trofimov 1 , Elena Yu. Schmidt 1
Affiliation
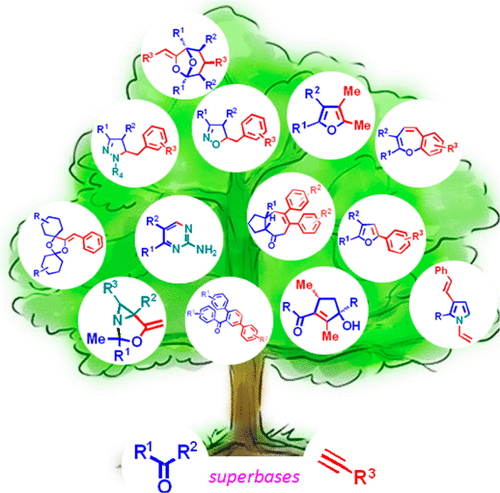
|
In this Account, we briefly discuss the recently discovered and rapidly developing superbase-promoted self-organization reactions of several equivalents of acetylenes and ketones to afford complex compounds that represent promising synthetic building blocks common in natural products. Notably, acetylenes play a special role in these reactions because of their dual (acting as an electrophile and a nucleophile) and flexible reactivity. These unique properties of acetylenes are elegantly expressed in superbasic media, where acetylenes are more deprotonated and their electrophilicity increases as a result of complexation with alkali metal cations, with simultaneous enhancement of the nucleophilic reactants due to desolvation. Under these conditions, acetylenes behave as a driving and organizing force toward other reactants. Various combinations of nucleophilic addition to the triple bond and acetylene deprotonation in the presence of other reactants with dual reactivity (e.g., ketones) enables the self-organization of complex molecular architectures that are inaccessible by conventional reactions. Herein we analyze recent achievements in this area concerning the reactions of acetylenes with ketones in superbasic KOH/DMSO-type systems that selectively afford synthetically and pharmaceutically valuable carbo- and heterocycles. Most of the reactions are triggered by the nucleophilic addition of deprotonated ketones (enolate anions) to acetylenes (superbase-catalyzed C-vinylation of ketones with acetylenes, which was recently introduced by our group into a toolkit of organic chemistry). The β,γ-ethylenic ketones thus formed can then take part in cascade processes with ketones and acetylenes to afford either carbocycles (e.g., hexahydroazulenones, acyl terphenyls, functionalized and cyclopentenols) or heterocycles (e.g., furans, benzoxepines, dioxabicyclo[3.2.1]octanes, and dioxadispiro[5.1.5.2]pentadecanes), depending on the structure of the reactants and the reaction conditions. Most of these compounds are selectively built from several equivalents of ketones and acetylenes in different combinations, and despite the presence of two or more asymmetric carbons in the products, they are generated as single diastereomers. When other nucleophiles (hydroxylamine, hydrazines, guanidine, and oximes) and ketones are involved in these self-organization processes, the intermediate β,γ-ethylenic ketones allow the formation of diverse heterocyclic systems (pyrroles, isoxazolines, pyrazolines, aminopyrimidines, and azabicyclo[3.1.0]hexanes). The discovered unique chemical transformations do not require transition metal catalysts and proceed under mild and operationally simple conditions. Most of these syntheses involve cascade addition reactions and therefore represent pot-, atom-, step-, and energy-saving processes that meet the requirements of green chemistry. The significance of the approach discussed herein is that it represents a viable alternative to existing classic and modern transition-metal-based catalytic syntheses of some fundamental carbo- and heterocycles. This is demonstrated by its employment of readily available, inexpensive starting materials like acetylenes and ketones and simple, widely accessible superbasic systems such as KOH/DMSO, which serves as a highly active universal catalyst and auxiliary. As shown in this Account, as this approach has developed, the number of preparatively attractive methods for the synthesis of diverse and potentially useful compounds has rapidly ballooned. The impressive experimental results presented in this Account will hopefully draw the attention of large circles of organic chemists involved in the design of rational and ecologically sound synthetic procedures and thus increase the application of these techniques in medicinal chemistry and materials science.
中文翻译:

乙炔在碳和杂环的超碱促进组装中
在本报告中,我们简要讨论了最近发现的,发展迅速的由超碱促进的,相当于乙炔和酮当量的自组织反应,以提供代表天然产品中常见的有前途的合成基础材料的复杂化合物。值得注意的是,由于乙炔的双重作用(充当亲电试剂和亲核试剂)和灵活的反应性,因此它们在这些反应中起着特殊的作用。乙炔的这些独特性质在超碱性介质中得到了很好的表达,其中乙炔更去质子化,由于与碱金属阳离子络合,其亲电性增加,同时由于去溶剂化而使亲核反应物同时增强。在这些条件下,乙炔充当对其他反应物的驱动力和组织力。在具有双反应性的其他反应物(例如酮)存在下,亲核加成至三键和乙炔去质子化的各种组合能够实现常规反应难以达到的复杂分子结构的自组织。本文中,我们分析了在超碱性KOH / DMSO型系统中乙炔与酮反应的最新进展,该系统可选择性提供合成和药学上有价值的碳环和杂环。大多数反应是由去质子化的酮(烯酸根阴离子)向乙炔的亲核加成(超碱催化的乙炔与酮的C-乙烯基化,这是我们小组最近引入的有机化学工具包)引发的。β,然后,如此形成的γ-乙烯酮可与酮和乙炔一起参与级联过程,从而提供碳环(例如六氢azulenones,酰基三联苯,官能化的和环戊烯醇)或杂环(例如呋喃,苯并xepines,二氧杂双环[3.2.1]辛烷,和二恶二螺[5.1.5.2]十五烷),取决于反应物的结构和反应条件。这些化合物中的大多数是由几种当量的酮和乙炔以不同的组合选择性地构建的,尽管产品中存在两个或多个不对称碳,它们仍以单一非对映异构体的形式生成。当其他亲核试剂(羟胺,肼,胍和肟)和酮参与这些自组织过程时,中间体β,γ-乙烯酮可形成各种杂环系统(吡咯,异恶唑啉,吡唑啉,氨基嘧啶和氮杂双环[3.1.0]己烷)。发现的独特化学转化不需要过渡金属催化剂,并且可以在温和且操作简单的条件下进行。这些合成中的大多数涉及级联加成反应,因此代表了满足绿色化学要求的罐,原子,步和节能过程。本文讨论的方法的意义在于,它代表了一些基本碳环和杂环的现有经典和现代基于过渡金属的催化合成方法的可行替代方案。通过使用容易获得的廉价起始原料(例如乙炔和酮)以及简单,广泛使用的超碱性系统,例如KOH / DMSO,可作为高活性通用催化剂和助剂。如该帐户中所示,随着这种方法的发展,用于合成各种可能有用的化合物的制备上有吸引力的方法迅速膨胀。该帐户中令人印象深刻的实验结果有望引起涉及合理和生态合理的合成程序设计的有机化学家的广泛关注,从而增加这些技术在药物化学和材料科学中的应用。合成各种可能有用的化合物的制备上有吸引力的方法的数量迅速增加。此帐户中令人印象深刻的实验结果有望引起参与合理和生态合理的合成程序设计的有机化学界的广泛关注,从而增加这些技术在药物化学和材料科学中的应用。合成各种可能有用的化合物的制备上有吸引力的方法的数量迅速增加。此帐户中令人印象深刻的实验结果有望引起参与合理和生态合理的合成程序设计的有机化学界的广泛关注,从而增加这些技术在药物化学和材料科学中的应用。
更新日期:2018-04-18
中文翻译:

乙炔在碳和杂环的超碱促进组装中
在本报告中,我们简要讨论了最近发现的,发展迅速的由超碱促进的,相当于乙炔和酮当量的自组织反应,以提供代表天然产品中常见的有前途的合成基础材料的复杂化合物。值得注意的是,由于乙炔的双重作用(充当亲电试剂和亲核试剂)和灵活的反应性,因此它们在这些反应中起着特殊的作用。乙炔的这些独特性质在超碱性介质中得到了很好的表达,其中乙炔更去质子化,由于与碱金属阳离子络合,其亲电性增加,同时由于去溶剂化而使亲核反应物同时增强。在这些条件下,乙炔充当对其他反应物的驱动力和组织力。在具有双反应性的其他反应物(例如酮)存在下,亲核加成至三键和乙炔去质子化的各种组合能够实现常规反应难以达到的复杂分子结构的自组织。本文中,我们分析了在超碱性KOH / DMSO型系统中乙炔与酮反应的最新进展,该系统可选择性提供合成和药学上有价值的碳环和杂环。大多数反应是由去质子化的酮(烯酸根阴离子)向乙炔的亲核加成(超碱催化的乙炔与酮的C-乙烯基化,这是我们小组最近引入的有机化学工具包)引发的。β,然后,如此形成的γ-乙烯酮可与酮和乙炔一起参与级联过程,从而提供碳环(例如六氢azulenones,酰基三联苯,官能化的和环戊烯醇)或杂环(例如呋喃,苯并xepines,二氧杂双环[3.2.1]辛烷,和二恶二螺[5.1.5.2]十五烷),取决于反应物的结构和反应条件。这些化合物中的大多数是由几种当量的酮和乙炔以不同的组合选择性地构建的,尽管产品中存在两个或多个不对称碳,它们仍以单一非对映异构体的形式生成。当其他亲核试剂(羟胺,肼,胍和肟)和酮参与这些自组织过程时,中间体β,γ-乙烯酮可形成各种杂环系统(吡咯,异恶唑啉,吡唑啉,氨基嘧啶和氮杂双环[3.1.0]己烷)。发现的独特化学转化不需要过渡金属催化剂,并且可以在温和且操作简单的条件下进行。这些合成中的大多数涉及级联加成反应,因此代表了满足绿色化学要求的罐,原子,步和节能过程。本文讨论的方法的意义在于,它代表了一些基本碳环和杂环的现有经典和现代基于过渡金属的催化合成方法的可行替代方案。通过使用容易获得的廉价起始原料(例如乙炔和酮)以及简单,广泛使用的超碱性系统,例如KOH / DMSO,可作为高活性通用催化剂和助剂。如该帐户中所示,随着这种方法的发展,用于合成各种可能有用的化合物的制备上有吸引力的方法迅速膨胀。该帐户中令人印象深刻的实验结果有望引起涉及合理和生态合理的合成程序设计的有机化学家的广泛关注,从而增加这些技术在药物化学和材料科学中的应用。合成各种可能有用的化合物的制备上有吸引力的方法的数量迅速增加。此帐户中令人印象深刻的实验结果有望引起参与合理和生态合理的合成程序设计的有机化学界的广泛关注,从而增加这些技术在药物化学和材料科学中的应用。合成各种可能有用的化合物的制备上有吸引力的方法的数量迅速增加。此帐户中令人印象深刻的实验结果有望引起参与合理和生态合理的合成程序设计的有机化学界的广泛关注,从而增加这些技术在药物化学和材料科学中的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号