当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heteroannulation of Arynes with α‐Amino Imides: Synthesis of 2,2‐Disubstituted Indolin‐3‐ones and Application to the Enantioselective Total Synthesis of (+)‐Hinckdentine A
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201800746 Rubén O. Torres-Ochoa 1 , Thomas Buyck 1 , Qian Wang 1 , Jieping Zhu 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201800746 Rubén O. Torres-Ochoa 1 , Thomas Buyck 1 , Qian Wang 1 , Jieping Zhu 1
Affiliation
|
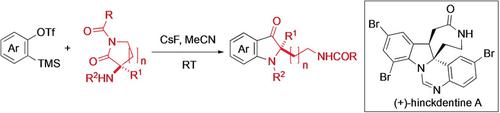
A novel heteroannulation reaction between α‐amino imides and in situ generated arynes has been developed for the synthesis of 2,2‐disubstituted indolin‐3‐ones. An enantioselective total synthesis of the marine alkaloid (+)‐hinckdentine A was subsequently accomplished using this reaction as a key step. A catalytic enantioselective Michael addition of an α‐aryl‐α‐isocyanoacetate to phenyl vinyl selenone was employed for the construction of the enantioenriched α‐quaternary α‐amino ester.
中文翻译:

带有α-氨基酰亚胺的芳烃的杂环化:2,2-二取代的吲哚-3-酮的合成及其对(+)-欣克汀丁A的对映选择性合成
已经开发了一种α-氨基酰亚胺和原位生成的芳烃之间的新型异环化反应,用于合成2,2-二取代的吲哚-3-酮。随后使用该反应作为关键步骤,完成了海洋生物碱(+)-hinckdentine A的对映选择性全合成。将α-芳基-α-异氰基乙酸酯的对映体选择性催化迈克尔加成到苯基乙烯基硒酮中,用于构建对映体富集的α-季铵α-氨基酯。
更新日期:2018-04-17
中文翻译:

带有α-氨基酰亚胺的芳烃的杂环化:2,2-二取代的吲哚-3-酮的合成及其对(+)-欣克汀丁A的对映选择性合成
已经开发了一种α-氨基酰亚胺和原位生成的芳烃之间的新型异环化反应,用于合成2,2-二取代的吲哚-3-酮。随后使用该反应作为关键步骤,完成了海洋生物碱(+)-hinckdentine A的对映选择性全合成。将α-芳基-α-异氰基乙酸酯的对映体选择性催化迈克尔加成到苯基乙烯基硒酮中,用于构建对映体富集的α-季铵α-氨基酯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号