当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identifying Catalytically Active Mononuclear Peroxoniobate Anion of Ionic Liquids in the Epoxidation of Olefins
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acscatal.7b04443 Wenbao Ma 1 , Haiyang Yuan 1 , Haifeng Wang 1 , Qingqing Zhou 1 , Kang Kong 1 , Difan Li 1 , Yefeng Yao 2 , Zhenshan Hou 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acscatal.7b04443 Wenbao Ma 1 , Haiyang Yuan 1 , Haifeng Wang 1 , Qingqing Zhou 1 , Kang Kong 1 , Difan Li 1 , Yefeng Yao 2 , Zhenshan Hou 1
Affiliation
|
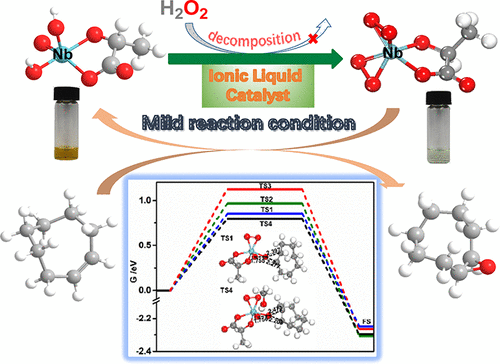
The organic carboxylic acid coordinated monomeric peroxoniobate-based ionic liquids (ILs) [TBA][NbO(OH)2(R)] (TBA = tetrabutylammonium; R = lactic acid (LA), glycolic acid (GLY), malic acid (MA)) were prepared and fully characterized by elemental analysis, NMR, IR, Raman, TGA, 93Nb NMR, and HRMS. These IL catalysts exhibited not only high catalytic activity for the epoxidation of olefins under very mild reaction conditions, as the turnover frequency of [TBA][NbO(OH)2(LA)] reached up to 110 h–1, but also satisfactory recyclability in the epoxidation by using only 1 equiv of hydrogen peroxide as an oxidant. Meanwhile, this work revealed that the ILs underwent structural transformation from [NbO(OH)2(R)]− to [Nb(O–O)2(R)]− (R = LA, GLY, MA) in the presence of H2O2 by a subsequent activity evaluation, characterization, and first-principles calculations. Moreover, the organic carboxylic acid coordinated monomeric peroxoniobate-based ILs were investigated using density functional theory (DFT) calculations, which identified that [Nb(O–O)2LA]− was more advantageous than [Nb(O–O)2(OOH)2]− for the epoxidation of olefins. Due to the coordination between the α-hydroxy acids and the monomeric peroxoniobate anions, the functionalized ILs can efficiently catalyze the epoxidation of a wide range of olefins and allylic alcohols under very mild conditions. Additionally, the effect of solvents on the reaction is illustrated. It was found that methanol can lower the epoxidation barriers by forming a hydrogen bond with a peroxo ligand attached to the niobium center.
中文翻译:

在烯烃环氧化中鉴定离子液体的催化活性单核过氧铌酸根阴离子
有机羧酸配位的过氧苯甲酸单体离子液体(TBs)[NbO(OH)2(R)](TBA =四丁基铵; R =乳酸(LA),乙醇酸(GLY),苹果酸(MA) ))制备并通过元素分析,NMR,IR,拉曼,TGA,93 Nb NMR和HRMS进行了全面表征。这些IL催化剂不仅在非常温和的反应条件下对烯烃的环氧化显示出高催化活性,因为[TBA] [NbO(OH)2(LA)]的周转频率达到110 h –1,而且还具有令人满意的可回收性仅使用1当量的过氧化氢作为氧化剂进行环氧化 同时,这项工作表明IL经历了[NbO(OH)2(R)]的结构转变。-通过后续的活性评估,表征和第一性原理计算,在H 2 O 2存在的情况下- [Nb(O-O)2(R)] -(R = LA,GLY,MA)。此外,使用密度泛函理论(DFT)计算研究了基于有机羧酸配位的单体过氧异氰酸酯的IL,它确定了[Nb(O-O)2 LA] -比[Nb(O-O)2( OOH)2 ] -用于烯烃的环氧化。由于α-羟基酸和单体过氧异氰酸酯阴离子之间的配位,官能化的ILs可以在非常温和的条件下有效催化各种烯烃和烯丙醇的环氧化。另外,还说明了溶剂对反应的影响。已经发现,甲醇可以通过与附着在铌中心的过氧配体形成氢键来降低环氧化势垒。
更新日期:2018-04-17
中文翻译:

在烯烃环氧化中鉴定离子液体的催化活性单核过氧铌酸根阴离子
有机羧酸配位的过氧苯甲酸单体离子液体(TBs)[NbO(OH)2(R)](TBA =四丁基铵; R =乳酸(LA),乙醇酸(GLY),苹果酸(MA) ))制备并通过元素分析,NMR,IR,拉曼,TGA,93 Nb NMR和HRMS进行了全面表征。这些IL催化剂不仅在非常温和的反应条件下对烯烃的环氧化显示出高催化活性,因为[TBA] [NbO(OH)2(LA)]的周转频率达到110 h –1,而且还具有令人满意的可回收性仅使用1当量的过氧化氢作为氧化剂进行环氧化 同时,这项工作表明IL经历了[NbO(OH)2(R)]的结构转变。-通过后续的活性评估,表征和第一性原理计算,在H 2 O 2存在的情况下- [Nb(O-O)2(R)] -(R = LA,GLY,MA)。此外,使用密度泛函理论(DFT)计算研究了基于有机羧酸配位的单体过氧异氰酸酯的IL,它确定了[Nb(O-O)2 LA] -比[Nb(O-O)2( OOH)2 ] -用于烯烃的环氧化。由于α-羟基酸和单体过氧异氰酸酯阴离子之间的配位,官能化的ILs可以在非常温和的条件下有效催化各种烯烃和烯丙醇的环氧化。另外,还说明了溶剂对反应的影响。已经发现,甲醇可以通过与附着在铌中心的过氧配体形成氢键来降低环氧化势垒。



























 京公网安备 11010802027423号
京公网安备 11010802027423号