当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nε-Acryloyllysine Piperazides as Irreversible Inhibitors of Transglutaminase 2: Synthesis, Structure–Activity Relationships, and Pharmacokinetic Profiling
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00286 Robert Wodtke 1, 2, 3 , Christoph Hauser 4 , Gloria Ruiz-Gómez 5 , Elisabeth Jäckel 1, 2 , David Bauer 1, 3 , Martin Lohse 1, 2 , Alan Wong 1 , Johanna Pufe 1 , Friedrich-Alexander Ludwig 6 , Steffen Fischer 6 , Sandra Hauser 1 , Dieter Greif 2 , M. Teresa Pisabarro 5 , Jens Pietzsch 1, 3 , Markus Pietsch 4 , Reik Löser 1, 3
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00286 Robert Wodtke 1, 2, 3 , Christoph Hauser 4 , Gloria Ruiz-Gómez 5 , Elisabeth Jäckel 1, 2 , David Bauer 1, 3 , Martin Lohse 1, 2 , Alan Wong 1 , Johanna Pufe 1 , Friedrich-Alexander Ludwig 6 , Steffen Fischer 6 , Sandra Hauser 1 , Dieter Greif 2 , M. Teresa Pisabarro 5 , Jens Pietzsch 1, 3 , Markus Pietsch 4 , Reik Löser 1, 3
Affiliation
|
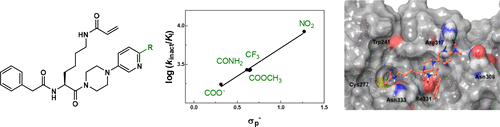
Transglutaminase 2 (TGase 2)-catalyzed transamidation represents an important post-translational mechanism for protein modification with implications in physiological and pathophysiological conditions, including fibrotic and neoplastic processes. Consequently, this enzyme is considered a promising target for the diagnosis of and therapy for these diseases. In this study, we report on the synthesis and kinetic characterization of Nε-acryloyllysine piperazides as irreversible inhibitors of TGase 2. Systematic structural modifications on 54 new compounds were performed with a major focus on fluorine-bearing substituents due to the potential of such compounds to serve as radiotracer candidates for positron emission tomography. The determined inhibitory activities ranged from 100 to 10 000 M–1 s–1, which resulted in comprehensive structure–activity relationships. Structure–activity correlations using various substituent parameters accompanied by covalent docking studies provide an advanced understanding of the molecular recognition for this inhibitor class within the active site of TGase 2. Selectivity profiling of selected compounds for other transglutaminases demonstrated an excellent selectivity toward transglutaminase 2. Furthermore, an initial pharmacokinetic profiling of selected inhibitors was performed, including the assessment of potential membrane permeability and liver microsomal stability.
中文翻译:

Ñ ε -Acryloyllysine Piperazides作为转谷氨酰胺酶2的不可逆抑制剂:合成,结构-活性关系,和药代动力学性能分析
转谷氨酰胺酶2(TGase 2)催化的转酰胺作用代表了重要的翻译后机制,可用于蛋白质修饰,影响生理和病理生理状况,包括纤维化和赘生性过程。因此,该酶被认为是诊断和治疗这些疾病的有希望的靶标。在这项研究中,我们对的合成和动力学鉴定报告Ñ ε -acryloyllysine piperazides为2. 54种新化合物系统的结构修改,用一大焦点上的含氟取代基的轴承进行转谷氨酰胺酶的不可逆抑制剂由于这种化合物的潜在用作正电子发射断层扫描的放射性示踪剂。确定的抑制活性为100至10000 M–1 s –1,这导致了全面的结构-活动关系。使用各种取代基参数进行结构-活性相关性并进行共价对接研究,使人们对TGase 2活性位点内该抑制剂类别的分子识别有了更深入的了解。所选化合物对其他转谷氨酰胺酶的选择性谱分析显示出对转谷氨酰胺酶2的优异选择性。 ,对所选抑制剂进行了初步药代动力学分析,包括评估潜在的膜通透性和肝微粒体稳定性。
更新日期:2018-04-17
中文翻译:

Ñ ε -Acryloyllysine Piperazides作为转谷氨酰胺酶2的不可逆抑制剂:合成,结构-活性关系,和药代动力学性能分析
转谷氨酰胺酶2(TGase 2)催化的转酰胺作用代表了重要的翻译后机制,可用于蛋白质修饰,影响生理和病理生理状况,包括纤维化和赘生性过程。因此,该酶被认为是诊断和治疗这些疾病的有希望的靶标。在这项研究中,我们对的合成和动力学鉴定报告Ñ ε -acryloyllysine piperazides为2. 54种新化合物系统的结构修改,用一大焦点上的含氟取代基的轴承进行转谷氨酰胺酶的不可逆抑制剂由于这种化合物的潜在用作正电子发射断层扫描的放射性示踪剂。确定的抑制活性为100至10000 M–1 s –1,这导致了全面的结构-活动关系。使用各种取代基参数进行结构-活性相关性并进行共价对接研究,使人们对TGase 2活性位点内该抑制剂类别的分子识别有了更深入的了解。所选化合物对其他转谷氨酰胺酶的选择性谱分析显示出对转谷氨酰胺酶2的优异选择性。 ,对所选抑制剂进行了初步药代动力学分析,包括评估潜在的膜通透性和肝微粒体稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号