当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
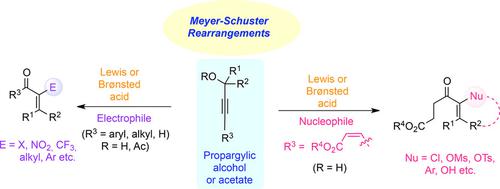
Intercepted Meyer–Schuster Rearrangements in Organic Synthesis
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-05-15 , DOI: 10.1002/ajoc.201800089 Debayan Roy 1 , Prabhakararao Tharra 1 , Beeraiah Baire 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-05-15 , DOI: 10.1002/ajoc.201800089 Debayan Roy 1 , Prabhakararao Tharra 1 , Beeraiah Baire 1
Affiliation
|
The Meyer–Schuster rearrangement of propargylic alcohols is a transformation in organic synthesis known for almost a century. The products of this reaction—α,β‐enones and their α‐functionalized units—are highly important functional groups for various synthetic transformations. Two modifications of this classical reaction, which involves interception of the intermediate allenol (or its equivalent) by either electrophiles or nucleophiles, have attracted the attention of synthetic organic chemists. This Focus Review provides a detailed description of the development of these two strategies.
中文翻译:

有机合成中截获的迈耶-舒斯特重排
炔丙醇的Meyer-Schuster重排是近一个世纪以来已知的有机合成方法的一种转变。该反应的产物-α,β-烯酮及其α-官能化单元-对于各种合成转化而言都是非常重要的官能团。对该经典反应的两种修饰涉及亲电试剂或亲核试剂拦截中间烯丙醇(或其等价物),引起了合成有机化学家的注意。本“重点回顾”详细介绍了这两种策略的发展。
更新日期:2018-05-15
中文翻译:

有机合成中截获的迈耶-舒斯特重排
炔丙醇的Meyer-Schuster重排是近一个世纪以来已知的有机合成方法的一种转变。该反应的产物-α,β-烯酮及其α-官能化单元-对于各种合成转化而言都是非常重要的官能团。对该经典反应的两种修饰涉及亲电试剂或亲核试剂拦截中间烯丙醇(或其等价物),引起了合成有机化学家的注意。本“重点回顾”详细介绍了这两种策略的发展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号