当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics and Mechanism of Oxirane Formation by Darzens Condensation of Ketones: Quantification of the Electrophilicities of Ketones
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-04-16 , DOI: 10.1021/jacs.8b01657 Zhen Li 1 , Harish Jangra 1 , Quan Chen 1 , Peter Mayer 1 , Armin R. Ofial 1 , Hendrik Zipse 1 , Herbert Mayr 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-04-16 , DOI: 10.1021/jacs.8b01657 Zhen Li 1 , Harish Jangra 1 , Quan Chen 1 , Peter Mayer 1 , Armin R. Ofial 1 , Hendrik Zipse 1 , Herbert Mayr 1
Affiliation
|
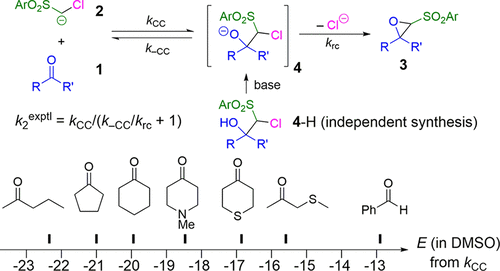
The kinetics of epoxide formation by Darzens condensation of aliphatic ketones 1 with arylsulfonyl-substituted chloromethyl anions 2 (ArSO2CHCl-) have been determined photometrically in DMSO solution at 20 °C. The reactions proceed via nucleophilic attack of the carbanions at the carbonyl group to give intermediate halohydrin anions 4, which subsequently cyclize with formation of the oxiranes 3. Protonation of the reaction mixture obtained in THF solution at low temperature allowed the intermediates to be trapped and the corresponding halohydrins 4-H to be isolated. Crossover experiments, i.e., deprotonation of the halohydrins 4-H in the presence of a trapping reagent for the regenerated arylsulfonyl-substituted chloromethyl anions 2, provided the relative rates of backward ( k-CC) and ring closure ( krc) reactions of the intermediates. Combination of the kinetic data ( k2exptl) with the splitting ratio ( k-CC/ krc) gave the second-order rate constants kCC for the attack of the carbanions 2 at the ketones 1. These kCC values and the previously reported reactivity parameters N and sN for the arylsulfonyl-substituted chloromethyl anions 2 allowed us to use the linear free energy relationship log k2(20 °C) = sN( N + E) for deriving the electrophilicity parameters E of the ketones 1 and thus predict potential nucleophilic reaction partners. Density functional theory calculations of the intrinsic reaction pathways showed that the reactions of the ketones 1 with the chloromethyl anions 2 yield two rotational isomers of the intermediate halohydrin anions 4, only one of which can cyclize while the other undergoes retroaddition because the barrier for rotation is higher than that for reversal to the reactants 1 and 2. The electrophilicity parameters E correlate moderately with the lowest unoccupied molecular orbital energies of the carbonyl groups, very poorly with Parr's electrophilicity indices, and best with the methyl anion affinities calculated for DMSO solution.
中文翻译:

酮的 Darzens 缩合形成环氧乙烷的动力学和机制:酮亲电性的量化
脂肪族酮 1 与芳基磺酰基取代的氯甲基阴离子 2 (ArSO2CHCl-) 的 Darzens 缩合形成环氧化物的动力学已在 20 °C 的 DMSO 溶液中通过光度计确定。反应通过羰基上的碳负离子的亲核攻击进行,得到中间体卤代醇阴离子 4,随后环化形成环氧乙烷 3。在低温下在 THF 溶液中获得的反应混合物的质子化允许中间体被捕获,并且相应的卤代醇 4-H 待分离。交叉实验,即在再生芳基磺酰基取代的氯甲基阴离子 2 的捕集剂存在下卤代醇 4-H 的去质子化,提供了中间体的反向 (k-CC) 和闭环 (krc) 反应的相对速率. 动力学数据 (k2exptl) 与分裂比 (k-CC/krc) 的组合给出了碳负离子 2 攻击酮 1 的二阶速率常数 kCC。这些 kCC 值和先前报道的反应性参数 N 和芳基磺酰基取代的氯甲基阴离子 2 的 sN 允许我们使用线性自由能关系 log k2(20 °C) = sN( N + E) 来推导酮 1 的亲电性参数 E,从而预测潜在的亲核反应伙伴。固有反应途径的密度泛函理论计算表明,酮 1 与氯甲基阴离子 2 的反应产生中间卤代醇阴离子 4 的两种旋转异构体,
更新日期:2018-04-16
中文翻译:

酮的 Darzens 缩合形成环氧乙烷的动力学和机制:酮亲电性的量化
脂肪族酮 1 与芳基磺酰基取代的氯甲基阴离子 2 (ArSO2CHCl-) 的 Darzens 缩合形成环氧化物的动力学已在 20 °C 的 DMSO 溶液中通过光度计确定。反应通过羰基上的碳负离子的亲核攻击进行,得到中间体卤代醇阴离子 4,随后环化形成环氧乙烷 3。在低温下在 THF 溶液中获得的反应混合物的质子化允许中间体被捕获,并且相应的卤代醇 4-H 待分离。交叉实验,即在再生芳基磺酰基取代的氯甲基阴离子 2 的捕集剂存在下卤代醇 4-H 的去质子化,提供了中间体的反向 (k-CC) 和闭环 (krc) 反应的相对速率. 动力学数据 (k2exptl) 与分裂比 (k-CC/krc) 的组合给出了碳负离子 2 攻击酮 1 的二阶速率常数 kCC。这些 kCC 值和先前报道的反应性参数 N 和芳基磺酰基取代的氯甲基阴离子 2 的 sN 允许我们使用线性自由能关系 log k2(20 °C) = sN( N + E) 来推导酮 1 的亲电性参数 E,从而预测潜在的亲核反应伙伴。固有反应途径的密度泛函理论计算表明,酮 1 与氯甲基阴离子 2 的反应产生中间卤代醇阴离子 4 的两种旋转异构体,



























 京公网安备 11010802027423号
京公网安备 11010802027423号