当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of Noncatalytic Lysine Residues from Allosteric Circuits via Covalent Probes
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1021/acschembio.8b00101 Jens Bongard 1 , Marian Lorenz 1 , Ingrid R. Vetter 2 , Patricia Stege 2 , Arthur T. Porfetye 2 , Anna Laura Schmitz 3 , Farnusch Kaschani 3 , Alex Wolf 4 , Uwe Koch 4 , Peter Nussbaumer 4 , Bert Klebl 4 , Markus Kaiser 3 , Michael Ehrmann 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1021/acschembio.8b00101 Jens Bongard 1 , Marian Lorenz 1 , Ingrid R. Vetter 2 , Patricia Stege 2 , Arthur T. Porfetye 2 , Anna Laura Schmitz 3 , Farnusch Kaschani 3 , Alex Wolf 4 , Uwe Koch 4 , Peter Nussbaumer 4 , Bert Klebl 4 , Markus Kaiser 3 , Michael Ehrmann 1
Affiliation
|
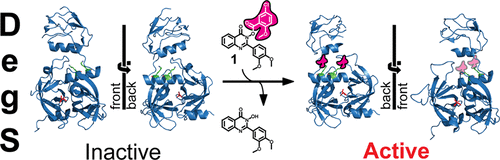
Covalent modifications of nonactive site lysine residues by small molecule probes has recently evolved into an important strategy for interrogating biological systems. Here, we report the discovery of a class of bioreactive compounds that covalently modify lysine residues in DegS, the rate limiting protease of the essential bacterial outer membrane stress response pathway. These modifications lead to an allosteric activation and allow the identification of novel residues involved in the allosteric activation circuit. These findings were validated by structural analyses via X-ray crystallography and cell-based reporter systems. We anticipate that our findings are not only relevant for a deeper understanding of the structural basis of allosteric activation in DegS and other HtrA serine proteases but also pinpoint an alternative use of covalent small molecules for probing essential biochemical mechanisms.
中文翻译:

通过共价探针从变构电路中鉴定非催化赖氨酸残基
小分子探针对非活性位点赖氨酸残基的共价修饰近来已发展成为询问生物系统的重要策略。在这里,我们报告发现了一类生物反应性化合物的发现,该化合物共价修饰DegS中的赖氨酸残基,DegS是必需细菌外膜应激反应途径的限速蛋白酶。这些修饰导致变构激活,并允许识别与变构激活电路有关的新残基。这些发现通过X射线晶体学和基于细胞的报告系统的结构分析得到了验证。
更新日期:2018-04-16
中文翻译:

通过共价探针从变构电路中鉴定非催化赖氨酸残基
小分子探针对非活性位点赖氨酸残基的共价修饰近来已发展成为询问生物系统的重要策略。在这里,我们报告发现了一类生物反应性化合物的发现,该化合物共价修饰DegS中的赖氨酸残基,DegS是必需细菌外膜应激反应途径的限速蛋白酶。这些修饰导致变构激活,并允许识别与变构激活电路有关的新残基。这些发现通过X射线晶体学和基于细胞的报告系统的结构分析得到了验证。



























 京公网安备 11010802027423号
京公网安备 11010802027423号