当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition and Regulation of the Ergothioneine Biosynthetic Methyltransferase EgtD
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1021/acschembio.8b00127 Laëtitia Misson 1 , Reto Burn 1 , Allegra Vit 2 , Julia Hildesheim 1 , Mariia A. Beliaeva 1 , Wulf Blankenfeldt 2, 3 , Florian P. Seebeck 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1021/acschembio.8b00127 Laëtitia Misson 1 , Reto Burn 1 , Allegra Vit 2 , Julia Hildesheim 1 , Mariia A. Beliaeva 1 , Wulf Blankenfeldt 2, 3 , Florian P. Seebeck 1
Affiliation
|
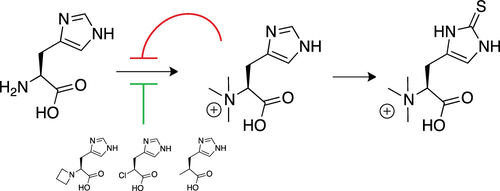
Ergothioneine is an emerging factor in cellular redox homeostasis in bacteria, fungi, plants, and animals. Reports that ergothioneine biosynthesis may be important for the pathogenicity of bacteria and fungi raise the question as to how this pathway is regulated and whether the corresponding enzymes may be therapeutic targets. The first step in ergothioneine biosynthesis is catalyzed by the methyltransferase EgtD that converts histidine into N-α-trimethylhistidine. This report examines the kinetic, thermodynamic and structural basis for substrate, product, and inhibitor binding by EgtD from Mycobacterium smegmatis. This study reveals an unprecedented substrate binding mechanism and a fine-tuned affinity landscape as determinants for product specificity and product inhibition. Both properties are evolved features that optimize the function of EgtD in the context of cellular ergothioneine production. On the basis of these findings, we developed a series of simple histidine derivatives that inhibit methyltransferase activity at low micromolar concentrations. Crystal structures of inhibited complexes validate this structure- and mechanism-based design strategy.
中文翻译:

麦角硫氨酸生物合成甲基转移酶EgtD的抑制与调控
麦角硫因是细菌,真菌,植物和动物中细胞氧化还原稳态的新兴因素。关于麦角硫因生物合成可能对细菌和真菌的致病性很重要的报道提出了关于如何调节该途径以及相应的酶是否可以作为治疗靶标的问题。麦角硫氨酸生物合成的第一步是由甲基转移酶EgtD催化,该酶将组氨酸转化为N-α-三甲基组氨酸。该报告研究了耻垢分枝杆菌的EgtD与底物,产物和抑制剂结合的动力学,热力学和结构基础。这项研究揭示了前所未有的底物结合机制和微调的亲和力景观,作为决定产品特异性和抑制产物的因素。两种特性都是在细胞麦角硫氨酸生产中优化EgtD功能的进化特征。基于这些发现,我们开发了一系列简单的组氨酸衍生物,它们在低微摩尔浓度下抑制甲基转移酶的活性。抑制复合物的晶体结构验证了这种基于结构和机理的设计策略。
更新日期:2018-04-16
中文翻译:

麦角硫氨酸生物合成甲基转移酶EgtD的抑制与调控
麦角硫因是细菌,真菌,植物和动物中细胞氧化还原稳态的新兴因素。关于麦角硫因生物合成可能对细菌和真菌的致病性很重要的报道提出了关于如何调节该途径以及相应的酶是否可以作为治疗靶标的问题。麦角硫氨酸生物合成的第一步是由甲基转移酶EgtD催化,该酶将组氨酸转化为N-α-三甲基组氨酸。该报告研究了耻垢分枝杆菌的EgtD与底物,产物和抑制剂结合的动力学,热力学和结构基础。这项研究揭示了前所未有的底物结合机制和微调的亲和力景观,作为决定产品特异性和抑制产物的因素。两种特性都是在细胞麦角硫氨酸生产中优化EgtD功能的进化特征。基于这些发现,我们开发了一系列简单的组氨酸衍生物,它们在低微摩尔浓度下抑制甲基转移酶的活性。抑制复合物的晶体结构验证了这种基于结构和机理的设计策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号