当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electric field mediated separation of water–ethanol mixtures in carbon-nanotubes integrated in nanoporous graphene membranes
Faraday Discussions ( IF 3.4 ) Pub Date : 2018-04-16 , DOI: 10.1039/c8fd00027a Manash Pratim Borthakur 1, 2, 3 , Dipankar Bandyopadhyay 2, 2, 3, 4, 5 , Gautam Biswas 1, 2, 3
Faraday Discussions ( IF 3.4 ) Pub Date : 2018-04-16 , DOI: 10.1039/c8fd00027a Manash Pratim Borthakur 1, 2, 3 , Dipankar Bandyopadhyay 2, 2, 3, 4, 5 , Gautam Biswas 1, 2, 3
Affiliation
|
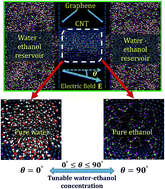
We investigate the influence of an applied electric field on the separation of a water–ethanol solution inside a carbon nanotube (CNT) using a series of molecular dynamics simulations. The electric field is applied at an angle θ with respect to the axis of the CNT. The study uncovers that with the application of a ‘small-angle’ electric field (e.g. smaller θ), the water molecules exhibit preferential occupancy inside the CNT, whereas the application of the same electric field at a ‘wide-angle’ mode (e.g. higher θ) fills the CNT with ethanol molecules in place of water. Remarkably, the direction of the electric field plays a pivotal role because the field exerts a contrasting influence on the behaviours of the water and ethanol molecules. The water dipoles are favourably aligned at small values of θ creating an ordered water structure inside the CNT. Increasing θ disrupts the water dipole orientation and leads to the preferential occupancy of the CNT by ethanol molecules. An in-depth analysis on the simulated systems unveil that, at lower values of θ, multiple layers of water molecules are physically adsorbed near the CNT walls, which is found to diminish as θ is increased. In comparison, at higher magnitudes of θ, the ethanol molecules are preferentially adsorbed inside the CNT. The average interaction energy per ethanol (water) molecule is found to increase (reduce) when θ is monotonically increased, which can be ascribed to the increase (decrease) in the intermolecular hydrogen bonding capacity of the ethanol (water) molecules at larger values of θ. Consequently, inside the CNT, the average occupancy of water molecules decreases and ethanol molecules increases, as θ is monotonically increased, leading to the separation of the ethanol–water mixture. The proposed methodology can convert an equimolar mixture (1 : 1) of ethanol–water into a concentrated one (14 : 1) when the electric field is applied orthogonal to the axis of the CNT. The separation efficiency is found to improve with an increase in the intensity of the externally applied electric field.
中文翻译:

电场介导的纳米多孔石墨烯膜中集成的碳纳米管中水-乙醇混合物的分离
我们使用一系列分子动力学模拟研究施加电场对碳纳米管(CNT)内部水-乙醇溶液分离的影响。相对于CNT的轴线以角度θ施加电场。研究发现,在“小角度”电场(例如较小的θ)的作用下,水分子在CNT内部表现出优先占有,而在“广角”模式下施加相同的电场(例如,更高的θ)用乙醇分子代替水填充CNT。值得注意的是,电场的方向起着举足轻重的作用,因为电场对水和乙醇分子的行为产生了相反的影响。水偶极子有利地以较小的θ对齐,从而在CNT内部创建有序的水结构。θ的增加会破坏水的偶极子方向,并导致乙醇分子优先占据CNT。对模拟系统的深入分析显示,在较低的θ值下,多层水分子物理吸附在CNT壁附近,并且发现随着θ的增加而减小。相比之下,在更高的θ值下因此,乙醇分子优先吸附在CNT内部。当θ单调增加时,发现每个乙醇(水)分子的平均相互作用能增加(减少),这可以归因于乙醇(水)分子的较大分子间氢键合能力的增加(减少)。θ。因此,随着θ的增加,在CNT内部,水分子的平均占有率下降,乙醇分子的上升。单调增加,导致乙醇-水混合物分离。当施加电场垂直于CNT的轴时,所提出的方法可以将乙醇/水的等摩尔混合物(1:1)转换为浓溶液(14:1)。发现分离效率随着外部施加电场强度的增加而提高。
更新日期:2018-09-28
中文翻译:

电场介导的纳米多孔石墨烯膜中集成的碳纳米管中水-乙醇混合物的分离
我们使用一系列分子动力学模拟研究施加电场对碳纳米管(CNT)内部水-乙醇溶液分离的影响。相对于CNT的轴线以角度θ施加电场。研究发现,在“小角度”电场(例如较小的θ)的作用下,水分子在CNT内部表现出优先占有,而在“广角”模式下施加相同的电场(例如,更高的θ)用乙醇分子代替水填充CNT。值得注意的是,电场的方向起着举足轻重的作用,因为电场对水和乙醇分子的行为产生了相反的影响。水偶极子有利地以较小的θ对齐,从而在CNT内部创建有序的水结构。θ的增加会破坏水的偶极子方向,并导致乙醇分子优先占据CNT。对模拟系统的深入分析显示,在较低的θ值下,多层水分子物理吸附在CNT壁附近,并且发现随着θ的增加而减小。相比之下,在更高的θ值下因此,乙醇分子优先吸附在CNT内部。当θ单调增加时,发现每个乙醇(水)分子的平均相互作用能增加(减少),这可以归因于乙醇(水)分子的较大分子间氢键合能力的增加(减少)。θ。因此,随着θ的增加,在CNT内部,水分子的平均占有率下降,乙醇分子的上升。单调增加,导致乙醇-水混合物分离。当施加电场垂直于CNT的轴时,所提出的方法可以将乙醇/水的等摩尔混合物(1:1)转换为浓溶液(14:1)。发现分离效率随着外部施加电场强度的增加而提高。


























 京公网安备 11010802027423号
京公网安备 11010802027423号