当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Diarylmethyl Sulfones by Palladium‐Catalyzed Enantioselective Benzylic Substitution: A Remarkable Effect of Water
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-04-14 , DOI: 10.1002/chem.201800744 Atifah Najib 1 , Koji Hirano 1 , Masahiro Miura 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-04-14 , DOI: 10.1002/chem.201800744 Atifah Najib 1 , Koji Hirano 1 , Masahiro Miura 1
Affiliation
|
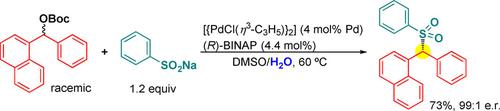
A Pd/(R)‐BINAP‐catalyzed enantioselective benzylic sulfonation of diarylmethyl carbonates with sodium sulfinates proceeds to deliver the corresponding chiral diarylmethyl sulfones in good yields with high enantioselectivity. The reaction occurs in a dynamic kinetic asymmetric transformation (DYKAT) manner and thus provides convergent access to optically active benzylic sulfones from racemic secondary benzylic carbonates. Additionally, the addition of H2O is found to be critical for high enantioselectivity.
中文翻译:

钯催化的对映选择性苯甲酸酯取代基不对称合成二芳基甲基砜:水的显着作用
Pd /(R)-BINAP催化的亚磺酸钠与碳酸二芳基甲基酯的对映选择性苄基磺化反应可以高收率和高对映选择性地提供相应的手性二芳基甲基砜。该反应以动态动力学不对称转化(DYKAT)方式发生,因此提供了从外消旋仲碳酸苄基酯到光学活性苄砜的会聚途径。另外,发现H 2 O的添加对于高对映选择性是至关重要的。
更新日期:2018-04-14
中文翻译:

钯催化的对映选择性苯甲酸酯取代基不对称合成二芳基甲基砜:水的显着作用
Pd /(R)-BINAP催化的亚磺酸钠与碳酸二芳基甲基酯的对映选择性苄基磺化反应可以高收率和高对映选择性地提供相应的手性二芳基甲基砜。该反应以动态动力学不对称转化(DYKAT)方式发生,因此提供了从外消旋仲碳酸苄基酯到光学活性苄砜的会聚途径。另外,发现H 2 O的添加对于高对映选择性是至关重要的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号