当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design of Mannose-Functionalized Curdlan Nanoparticles for Macrophage-Targeted siRNA Delivery
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2018-04-12 00:00:00 , DOI: 10.1021/acsami.8b02073 Tsogzolmaa Ganbold 1 , Huricha Baigude 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2018-04-12 00:00:00 , DOI: 10.1021/acsami.8b02073 Tsogzolmaa Ganbold 1 , Huricha Baigude 1
Affiliation
|
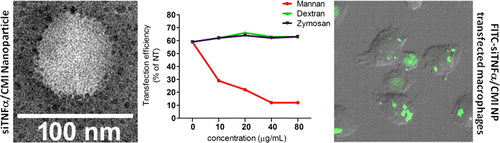
6-Amino-6-deoxy-curdlan is a promising nucleic acid carrier that efficiently delivers plasmid DNA as well as short interfering RNA (siRNA) to various cell lines. The highly reactive C6-NH2 groups of 6-amino-6-deoxy-curdlan prompt conjugation of various side groups including tissue-targeting ligands to enhance cell-type-specific nucleic acid delivery to specific cell lines. Herein, to test the primary-cell-targeting efficiency of the curdlan derivative, we chemically conjugated a macrophage-targeting ligand, mannose, to 6-amino-6-deoxy-curdlan. The resulting curdlan derivative (denoted CMI) readily complexed with siRNA and formed nanoparticles with a diameter of 50–80 nm. The CMI nanoparticles successfully delivered a dye-labeled siRNA to mouse peritoneal macrophages. The delivery efficiency was blocked by mannan, a natural ligand for a macrophage surface mannose receptor (CD206), but not by zymosan, a ligand for the dectin-1 receptor, which is also present on the surface of macrophages. Moreover, CMI nanoparticles were internalized by macrophages only at 37 °C, suggesting that the cellular uptake of CMI nanoparticles was energy-dependent. Furthermore, CMI nanoparticle efficiently delivered siRNA against tumor necrosis factor α (TNFα) to lipopolysaccharide-stimulated primary mouse peritoneal macrophages. In vivo experiments demonstrated that CMI nanoparticles successfully delivered siTNFα to mouse peritoneal macrophages, liver, and lung and induced significant knockdown of the TNFα expression at both messenger RNA and protein levels. Therefore, our design of CMI may be a promising siRNA carrier for targeting CD206-expressing primary cells such as macrophage and dendritic cells.
中文翻译:

甘露糖功能化的柯德兰纳米粒子的设计为巨噬细胞靶向的siRNA交付。
6-氨基-6-脱氧curdlan是一种很有前途的核酸载体,可以有效地将质粒DNA以及短干扰RNA(siRNA)输送至各种细胞系。高反应性C6-NH 2一组6-氨基-6-脱氧-柯德兰蛋白可促进各种侧基的结合,包括靶向组织的配体,以增强细胞类型特异性核酸向特定细胞系的传递。在本文中,为了测试柯德兰衍生物的原代细胞靶向效率,我们将靶向巨噬细胞的配体甘露糖化学缀合至6-氨基-6-脱氧柯德兰。所得的柯德兰衍生物(表示为CMI)易于与siRNA络合并形成直径为50-80 nm的纳米颗粒。CMI纳米粒子成功地将染料标记的siRNA递送至小鼠腹膜巨噬细胞。递送效率被甘露聚糖(一种巨噬细胞表面甘露糖受体(CD206)的天然配体)阻断,但未被zymosan(一种与dectin-1受体的配体结合)阻断,后者也存在于巨噬细胞表面。而且,CMI纳米颗粒仅在37°C时才被巨噬细胞内化,这表明CMI纳米颗粒的细胞摄取是能量依赖的。此外,CMI纳米颗粒有效地将针对肿瘤坏死因子α(TNFα)的siRNA传递给脂多糖刺激的原代小鼠腹膜巨噬细胞。体内实验表明,CMI纳米颗粒成功地将siTNFα递送至小鼠腹膜巨噬细胞,肝脏和肺部,并在信使RNA和蛋白质水平上均诱导了TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。CMI纳米颗粒有效地将针对肿瘤坏死因子α(TNFα)的siRNA传递至脂多糖刺激的原代小鼠腹膜巨噬细胞。体内实验表明,CMI纳米颗粒成功地将siTNFα递送至小鼠腹膜巨噬细胞,肝脏和肺部,并在信使RNA和蛋白质水平上均诱导了TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。CMI纳米颗粒有效地将针对肿瘤坏死因子α(TNFα)的siRNA传递至脂多糖刺激的原代小鼠腹膜巨噬细胞。体内实验表明,CMI纳米颗粒成功地将siTNFα递送至小鼠腹膜巨噬细胞,肝脏和肺部,并在信使RNA和蛋白质水平上均诱导了TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。并在信使RNA和蛋白质水平上都诱导TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。并在信使RNA和蛋白质水平上都诱导TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。
更新日期:2018-04-12
中文翻译:

甘露糖功能化的柯德兰纳米粒子的设计为巨噬细胞靶向的siRNA交付。
6-氨基-6-脱氧curdlan是一种很有前途的核酸载体,可以有效地将质粒DNA以及短干扰RNA(siRNA)输送至各种细胞系。高反应性C6-NH 2一组6-氨基-6-脱氧-柯德兰蛋白可促进各种侧基的结合,包括靶向组织的配体,以增强细胞类型特异性核酸向特定细胞系的传递。在本文中,为了测试柯德兰衍生物的原代细胞靶向效率,我们将靶向巨噬细胞的配体甘露糖化学缀合至6-氨基-6-脱氧柯德兰。所得的柯德兰衍生物(表示为CMI)易于与siRNA络合并形成直径为50-80 nm的纳米颗粒。CMI纳米粒子成功地将染料标记的siRNA递送至小鼠腹膜巨噬细胞。递送效率被甘露聚糖(一种巨噬细胞表面甘露糖受体(CD206)的天然配体)阻断,但未被zymosan(一种与dectin-1受体的配体结合)阻断,后者也存在于巨噬细胞表面。而且,CMI纳米颗粒仅在37°C时才被巨噬细胞内化,这表明CMI纳米颗粒的细胞摄取是能量依赖的。此外,CMI纳米颗粒有效地将针对肿瘤坏死因子α(TNFα)的siRNA传递给脂多糖刺激的原代小鼠腹膜巨噬细胞。体内实验表明,CMI纳米颗粒成功地将siTNFα递送至小鼠腹膜巨噬细胞,肝脏和肺部,并在信使RNA和蛋白质水平上均诱导了TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。CMI纳米颗粒有效地将针对肿瘤坏死因子α(TNFα)的siRNA传递至脂多糖刺激的原代小鼠腹膜巨噬细胞。体内实验表明,CMI纳米颗粒成功地将siTNFα递送至小鼠腹膜巨噬细胞,肝脏和肺部,并在信使RNA和蛋白质水平上均诱导了TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。CMI纳米颗粒有效地将针对肿瘤坏死因子α(TNFα)的siRNA传递至脂多糖刺激的原代小鼠腹膜巨噬细胞。体内实验表明,CMI纳米颗粒成功地将siTNFα递送至小鼠腹膜巨噬细胞,肝脏和肺部,并在信使RNA和蛋白质水平上均诱导了TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。并在信使RNA和蛋白质水平上都诱导TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。并在信使RNA和蛋白质水平上都诱导TNFα表达的显着降低。因此,我们对CMI的设计可能是有针对性的siRNA载体,可用于靶向表达CD206的原代细胞,例如巨噬细胞和树突状细胞。



























 京公网安备 11010802027423号
京公网安备 11010802027423号