当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of N-Alkyl Piperazine Side Chain Based CXCR4 Antagonists with Improved Drug-like Properties
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00030 Yesim A. Tahirovic 1 , Valarie M. Truax 1 , Robert J. Wilson 1 , Edgars Jecs 1 , Huy H. Nguyen 1 , Eric J. Miller 1 , Michelle B. Kim 1 , Katie M. Kuo 1 , Tao Wang 2 , Chi S. Sum 2 , Mary E. Cvijic 2 , Gretchen M. Schroeder 2 , Lawrence J. Wilson 1 , Dennis C. Liotta 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00030 Yesim A. Tahirovic 1 , Valarie M. Truax 1 , Robert J. Wilson 1 , Edgars Jecs 1 , Huy H. Nguyen 1 , Eric J. Miller 1 , Michelle B. Kim 1 , Katie M. Kuo 1 , Tao Wang 2 , Chi S. Sum 2 , Mary E. Cvijic 2 , Gretchen M. Schroeder 2 , Lawrence J. Wilson 1 , Dennis C. Liotta 1
Affiliation
|
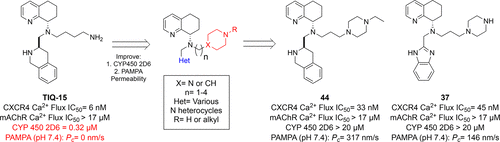
A novel series of CXCR4 antagonists with piperidinyl and piperazinyl alkylamine side chains designed as butyl amine replacements are described. Several of these compounds showed similar activity to the parent compound TIQ-15 (5) in a SDF-1 induced calcium flux assay. Preliminary structure–activity relationship investigations led us to identify a series containing N-propyl piperazine side chain analogs exemplified by 16 with improved off-target effects as measured in a muscarinic acetylcholine receptor (mAChR) calcium flux assay and in a limited drug safety panel screen. Further efforts to explore SAR and optimize drug properties led to the identification of the N′-ethyl-N-propyl-piperazine tetrahydroisoquinoline derivative 44 and the N-propyl-piperazine benzimidazole compound 37, which gave the best overall profiles with no mAChR or CYP450 inhibition, good permeability in PAMPA assays, and metabolic stability in human liver microsomes.
中文翻译:

发现基于N-烷基哌嗪侧链的CXCR4拮抗剂,具有改善的类药物特性
描述了具有设计成丁胺替代物的具有哌啶基和哌嗪基烷基胺侧链的一系列新的CXCR4拮抗剂。这些化合物中的几种在SDF-1诱导的钙通量测定中显示出与母体化合物TIQ-15(5)相似的活性。初步的结构-活性关系研究使我们确定了一个含有N-丙基哌嗪侧链类似物的系列,其中以16个为例,在毒蕈碱乙酰胆碱受体(mAChR)钙通量测定和有限的药物安全性面板筛选中测得的脱靶效应得到改善。进一步探索SAR和优化药物特性的努力导致了N'-乙基-N的鉴定-丙基-哌嗪四氢异喹啉衍生物44和N-丙基-哌嗪苯并咪唑化合物37具有最佳的总体特征,无mAChR或CYP450抑制作用,在PAMPA分析中具有良好的渗透性,在人肝微粒体中具有代谢稳定性。
更新日期:2018-04-09
中文翻译:

发现基于N-烷基哌嗪侧链的CXCR4拮抗剂,具有改善的类药物特性
描述了具有设计成丁胺替代物的具有哌啶基和哌嗪基烷基胺侧链的一系列新的CXCR4拮抗剂。这些化合物中的几种在SDF-1诱导的钙通量测定中显示出与母体化合物TIQ-15(5)相似的活性。初步的结构-活性关系研究使我们确定了一个含有N-丙基哌嗪侧链类似物的系列,其中以16个为例,在毒蕈碱乙酰胆碱受体(mAChR)钙通量测定和有限的药物安全性面板筛选中测得的脱靶效应得到改善。进一步探索SAR和优化药物特性的努力导致了N'-乙基-N的鉴定-丙基-哌嗪四氢异喹啉衍生物44和N-丙基-哌嗪苯并咪唑化合物37具有最佳的总体特征,无mAChR或CYP450抑制作用,在PAMPA分析中具有良好的渗透性,在人肝微粒体中具有代谢稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号