当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High GC Content Cas9-Mediated Genome-Editing and Biosynthetic Gene Cluster Activation in Saccharopolyspora erythraea
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acssynbio.7b00448 Yong Liu 1 , Wen-Ping Wei 1 , Bang-Ce Ye 1, 2
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acssynbio.7b00448 Yong Liu 1 , Wen-Ping Wei 1 , Bang-Ce Ye 1, 2
Affiliation
|
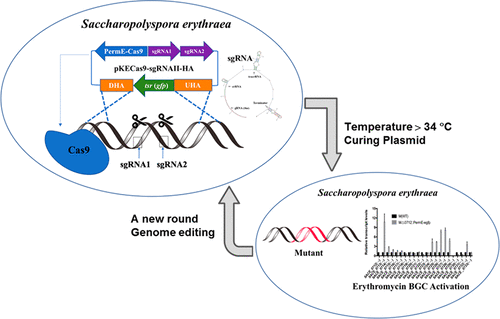
The overexpression of bacterial secondary metabolite biosynthetic enzymes is the basis for industrial overproducing strains. Genome editing tools can be used to further improve gene expression and yield. Saccharopolyspora erythraea produces erythromycin, which has extensive clinical applications. In this study, the CRISPR-Cas9 system was used to edit genes in the S. erythraea genome. A temperature-sensitive plasmid containing the PermE promoter, to drive Cas9 expression, and the Pj23119 and PkasO promoters, to drive sgRNAs, was designed. Erythromycin esterase, encoded by S. erythraea SACE_1765, inactivates erythromycin by hydrolyzing the macrolactone ring. Sequencing and qRT-PCR confirmed that reporter genes were successfully inserted into the SACE_1765 gene. Deletion of SACE_1765 in a high-producing strain resulted in a 12.7% increase in erythromycin levels. Subsequent PermE-egfp knock-in at the SACE_0712 locus resulted in an 80.3% increase in erythromycin production compared with that of wild type. Further investigation showed that PermE promoter knock-in activated the erythromycin biosynthetic gene clusters at the SACE_0712 locus. Additionally, deletion of indA (SACE_1229) using dual sgRNA targeting without markers increased the editing efficiency to 65%. In summary, we have successfully applied Cas9-based genome editing to a bacterial strain, S. erythraea, with a high GC content. This system has potential application for both genome-editing and biosynthetic gene cluster activation in Actinobacteria.
中文翻译:

高糖含量的Cas9介导的基因组编辑和生物合成基因簇激活的红糖酵母。
细菌次级代谢产物生物合成酶的过表达是工业生产菌株的基础。基因组编辑工具可用于进一步改善基因表达和产量。糖多孢菌(Saccharopolyspora erythraea)产生红霉素,其具有广泛的临床应用。在这项研究中,CRISPR-Cas9系统被用于编辑S. erythraea基因组中的基因。设计了一个温度敏感的质粒,该质粒包含驱动Cas9表达的PermE启动子,以及驱动sgRNA的Pj23119和PkasO启动子。红霉素酯酶,由S. erythraea编码SACE_1765通过水解大内酯环使红霉素失活。测序和qRT-PCR证实报告基因已成功插入SACE_1765基因。高产菌株中SACE_1765的删除导致红霉素水平增加12.7%。与野生型相比,随后在SACE_0712位点进行的PermE- egfp敲入导致红霉素产量增加了80.3%。进一步的研究表明,PermE启动子敲入激活了SACE_0712位点的红霉素生物合成基因簇。此外,使用无标记的双重sgRNA靶向缺失indA(SACE_1229)可将编辑效率提高到65%。总而言之,我们已经成功地将基于Cas9的基因组编辑应用于细菌菌株,S. erythraea,GC含量高。该系统对于放线菌中的基因组编辑和生物合成基因簇激活都有潜在的应用。
更新日期:2018-04-10
中文翻译:

高糖含量的Cas9介导的基因组编辑和生物合成基因簇激活的红糖酵母。
细菌次级代谢产物生物合成酶的过表达是工业生产菌株的基础。基因组编辑工具可用于进一步改善基因表达和产量。糖多孢菌(Saccharopolyspora erythraea)产生红霉素,其具有广泛的临床应用。在这项研究中,CRISPR-Cas9系统被用于编辑S. erythraea基因组中的基因。设计了一个温度敏感的质粒,该质粒包含驱动Cas9表达的PermE启动子,以及驱动sgRNA的Pj23119和PkasO启动子。红霉素酯酶,由S. erythraea编码SACE_1765通过水解大内酯环使红霉素失活。测序和qRT-PCR证实报告基因已成功插入SACE_1765基因。高产菌株中SACE_1765的删除导致红霉素水平增加12.7%。与野生型相比,随后在SACE_0712位点进行的PermE- egfp敲入导致红霉素产量增加了80.3%。进一步的研究表明,PermE启动子敲入激活了SACE_0712位点的红霉素生物合成基因簇。此外,使用无标记的双重sgRNA靶向缺失indA(SACE_1229)可将编辑效率提高到65%。总而言之,我们已经成功地将基于Cas9的基因组编辑应用于细菌菌株,S. erythraea,GC含量高。该系统对于放线菌中的基因组编辑和生物合成基因簇激活都有潜在的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号