当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substrate Recognition by a Colistin Resistance Enzyme from Moraxella catarrhalis
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acschembio.8b00116 Peter J. Stogios 1 , Georgina Cox 2 , Haley L. Zubyk 2 , Elena Evdokimova 1 , Zdzislaw Wawrzak 3 , Gerard D. Wright 2 , Alexei Savchenko 1, 4
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acschembio.8b00116 Peter J. Stogios 1 , Georgina Cox 2 , Haley L. Zubyk 2 , Elena Evdokimova 1 , Zdzislaw Wawrzak 3 , Gerard D. Wright 2 , Alexei Savchenko 1, 4
Affiliation
|
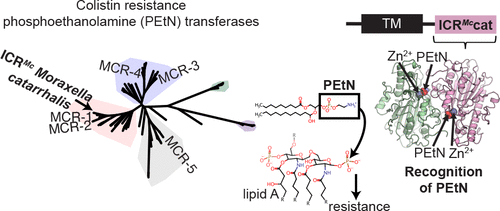
Lipid A phosphoethanolamine (PEtN) transferases render bacteria resistant to the last resort antibiotic colistin. The recent discoveries of pathogenic bacteria harboring plasmid-borne PEtN transferase (mcr) genes have illustrated the serious potential for wide dissemination of these resistance elements. The origin of mcr-1 is traced to Moraxella species co-occupying environmental niches with Enterobacteriaceae. Here, we describe the crystal structure of the catalytic domain of the chromosomally encoded colistin resistance PEtN transferase, ICRMc (for intrinsic colistin resistance) of Moraxella catarrhalis. The ICRMc structure in complex with PEtN reveals key molecular details including specific residues involved in catalysis and PEtN binding. It also demonstrates that ICRMc catalytic domain dimerization is required for substrate binding. Our structure-guided phylogenetic analysis provides sequence signatures defining potentially colistin-active representatives in this enzyme family. Combined, these results advance the molecular and mechanistic understanding of PEtN transferases and illuminate their origins.
中文翻译:

卡他莫拉氏菌的共利斯汀抗性酶对底物的识别
脂质A磷酸乙醇胺(PEtN)转移酶使细菌对最后一种抗生素大肠菌素具有抗性。携带质粒携带的PEtN转移酶(mcr)基因的致病细菌的最新发现说明了广泛传播这些抗性元件的巨大潜力。mcr-1的起源可追溯到与肠杆菌科共同占据环境壁Mor的莫拉氏菌物种。在这里,我们描述了卡他莫拉氏菌的染色体编码大肠菌素抗性PEtN转移酶,ICR Mc(内在大肠菌素抗性)的催化结构域的晶体结构。ICR Mc与PEtN结合的分子结构揭示了关键的分子细节,包括参与催化和PEtN结合的特定残基。它也证明了ICR Mc催化结构域二聚化是底物结合所必需的。我们的结构指导系统发育分析提供了定义该酶家族中潜在的大肠菌素活性代表的序列特征。结合起来,这些结果提高了对PEtN转移酶的分子和机理的理解,并阐明了它们的起源。
更新日期:2018-04-09
中文翻译:

卡他莫拉氏菌的共利斯汀抗性酶对底物的识别
脂质A磷酸乙醇胺(PEtN)转移酶使细菌对最后一种抗生素大肠菌素具有抗性。携带质粒携带的PEtN转移酶(mcr)基因的致病细菌的最新发现说明了广泛传播这些抗性元件的巨大潜力。mcr-1的起源可追溯到与肠杆菌科共同占据环境壁Mor的莫拉氏菌物种。在这里,我们描述了卡他莫拉氏菌的染色体编码大肠菌素抗性PEtN转移酶,ICR Mc(内在大肠菌素抗性)的催化结构域的晶体结构。ICR Mc与PEtN结合的分子结构揭示了关键的分子细节,包括参与催化和PEtN结合的特定残基。它也证明了ICR Mc催化结构域二聚化是底物结合所必需的。我们的结构指导系统发育分析提供了定义该酶家族中潜在的大肠菌素活性代表的序列特征。结合起来,这些结果提高了对PEtN转移酶的分子和机理的理解,并阐明了它们的起源。


























 京公网安备 11010802027423号
京公网安备 11010802027423号