当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of a Salt Coformer in a Co-Amorphous Drug System Dramatically Enhances the Glass Transition Temperature: A Case Study of the Ternary System Carbamazepine, Citric Acid, and l-Arginine
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00174 Hiroshi Ueda 1, 2 , Wenqi Wu 1 , Korbinian Löbmann 1 , Holger Grohganz 1 , Anette Müllertz 1 , Thomas Rades 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00174 Hiroshi Ueda 1, 2 , Wenqi Wu 1 , Korbinian Löbmann 1 , Holger Grohganz 1 , Anette Müllertz 1 , Thomas Rades 1
Affiliation
|
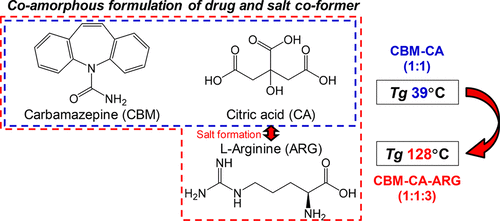
The use of co-amorphous systems containing a combination of low molecular weight drugs and excipients is a relatively new technology in the pharmaceutical field to improve the solubility of poorly water-soluble drugs. However, some co-amorphous systems show a lower glass transition temperature (Tg) than many of their polymeric solid dispersion counterparts. In this study, we aimed at designing a stable co-amorphous system with an elevated Tg. Carbamazepine (CBM) and citric acid (CA) were employed as the model drug and the coformer, respectively. co-amorphous CBM–CA at a 1:1 molar ratio was formed by ball milling, but a transition from the glassy to the supercooled melt state was observed under ambient conditions, due to the relatively low Tg of 38.8 °C of the co-amorphous system and moisture absorption. To improve the Tg of the coformer, salt formation of a combination of l-arginine (ARG) with CA was studied. First, ball milling of CA–ARG at molar ratios of 1:1, 1:2, and 1:3 forming co-amorphous systems was performed and led to a dramatic enhancement of the Tg, depending on the CA–ARG ratio. Salt formation between CA and ARG was observed by infrared spectroscopy. Next, ball milling of CBM–CA–ARG at molar ratios of 1:1:1, 1:1:2, and 1:1:3 resulted in co-amorphous blends, which had a single Tg at 77.8, 105.3, and 127.8 °C, respectively. These ternary co-amorphous samples remained in a solid amorphous form for 2 months at 40 °C. From these results, it can be concluded that blending of the salt coformer with a drug is a promising strategy to design stable co-amorphous formulations.
中文翻译:

盐共聚体在共非晶体系中的应用显着提高了玻璃化转变温度:以三元体系卡马西平,柠檬酸和l-精氨酸为例
使用包含低分子量药物和赋形剂组合的共非晶体系是药学领域中相对较新的技术,可提高水溶性差的药物的溶解度。但是,某些共非晶体系的玻璃化转变温度(T g)低于许多其对应的聚合物固体分散体。在这项研究中,我们旨在设计一个具有升高的T g的稳定的共非晶体系。卡马西平(CBM)和柠檬酸(CA)分别用作模型药物和共形成剂。通过球磨形成摩尔比为1:1的共非晶CBM-CA,但是由于相对较低的T,在环境条件下观察到了从玻璃态到过冷熔融态的转变g为38.8°C的共非晶体系和吸湿性。为了改善共形成剂的T g,研究了1-精氨酸(ARG)与CA的组合的盐形成。首先,对CA–ARG以1:1、1:2和1:3的摩尔比形成共非晶体系进行球磨,并根据CA–ARG的比例导致T g显着提高。通过红外光谱观察到CA和ARG之间的盐形成。接下来,以1:1:1、1:1:2和1:1:3的摩尔比对CBM–CA–ARG进行球磨,得到共非晶混合物,其单个T g分别在77.8、105.3和127.8°C下进行。这些三元共非晶样品在40°C下以固体非晶形式保留2个月。从这些结果可以得出结论,将盐共形成剂与药物共混是设计稳定的共无定形制剂的有前途的策略。
更新日期:2018-04-09
中文翻译:

盐共聚体在共非晶体系中的应用显着提高了玻璃化转变温度:以三元体系卡马西平,柠檬酸和l-精氨酸为例
使用包含低分子量药物和赋形剂组合的共非晶体系是药学领域中相对较新的技术,可提高水溶性差的药物的溶解度。但是,某些共非晶体系的玻璃化转变温度(T g)低于许多其对应的聚合物固体分散体。在这项研究中,我们旨在设计一个具有升高的T g的稳定的共非晶体系。卡马西平(CBM)和柠檬酸(CA)分别用作模型药物和共形成剂。通过球磨形成摩尔比为1:1的共非晶CBM-CA,但是由于相对较低的T,在环境条件下观察到了从玻璃态到过冷熔融态的转变g为38.8°C的共非晶体系和吸湿性。为了改善共形成剂的T g,研究了1-精氨酸(ARG)与CA的组合的盐形成。首先,对CA–ARG以1:1、1:2和1:3的摩尔比形成共非晶体系进行球磨,并根据CA–ARG的比例导致T g显着提高。通过红外光谱观察到CA和ARG之间的盐形成。接下来,以1:1:1、1:1:2和1:1:3的摩尔比对CBM–CA–ARG进行球磨,得到共非晶混合物,其单个T g分别在77.8、105.3和127.8°C下进行。这些三元共非晶样品在40°C下以固体非晶形式保留2个月。从这些结果可以得出结论,将盐共形成剂与药物共混是设计稳定的共无定形制剂的有前途的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号