Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2018-04-07 , DOI: 10.1016/j.cej.2018.04.047 Dan Zhang , Langping Wu , Jun Yao , Hartmut Herrmann , Hans-Hermann Richnow
|
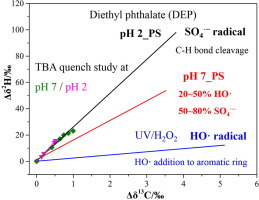
This study investigated 13C and 2H isotope fractionation associated with oxidation of three phthalate esters (PAEs) by radical species, including sulfate radical (SO4−) induced by heat-activated persulfate (PS) and hydroxyl radical (HO
) induced by UV/H2O2. For persulfate oxidation at pH = 2 and pH = 7, similar carbon isotope fractionation (εC) but distinct hydrogen isotope enrichment factors (εH) were observed. The UV/H2O2 reaction of three PAEs showed smaller εH values in comparison with persulfate oxidation. The correlation of 2H and 13C fractionation (Λ) allows to distinguish the persulfate oxidation (25.7 ± 2.6) and UV/H2O2 oxidation (2.4 ± 0.2) of diethyl phthalate (DEP) highlighting the potential of compound-specific stable isotope analysis (CSIA) to characterize chemical oxidation mechanism of PAEs. Moreover, study of radical quenching and CSIA were combined to explore the dominant radical species during persulfate oxidation of DEP. SO4
− was found to be the predominant radical at pH = 2. Both SO4
− and HO
contributed to DEP degradation at pH = 7 and HO
was estimated to have a contribution of 21–63% according to dual C
H isotope fractionation values. Carbon and hydrogen apparent kinetic isotope effects (AKIEs) (13C-AKIE = 1.017, 2H-AKIE = 2.41) obtained from dominating sulfate radical reaction of DEP both supported the hypothesis of C
H bond cleavage. Thus, carbon and hydrogen isotope enrichment factors clearly distinguish the different reaction mechanisms and hence, are a promising approach to improve understanding of radical species reaction pathways for chemical oxidation of PAEs.
中文翻译:

硫酸盐和羟基自由基降解过程中邻苯二甲酸酯的碳和氢同位素分馏
本实验研究13 C和2用三个邻苯二甲酸酯(邻苯二甲酸酯)通过自由基物种,包括氧化相关氢同位素分馏硫酸根(SO 4 - )诱导热活化过硫酸盐(PS)和羟基自由基(HO
)诱导的UV / H 2 O 2。在pH = 2和pH = 7,类似碳同位素分馏(ε过氧化Ç),但不同的氢同位素富集因子(ε ħ)观察。所述UV / H 2 ö 2三种邻苯二甲酸酯反应显示较小的ε ħ与过硫酸盐氧化的比较值。2的相关H和13 C分馏(Λ)可以区分邻苯二甲酸二乙酯(DEP)的过硫酸盐氧化(25.7±2.6)和UV / H 2 O 2氧化(2.4±0.2),突出了化合物特异性稳定同位素分析(CSIA)的潜力)表征PAE的化学氧化机理。此外,结合了自由基猝灭和CSIA的研究,以探索DEP过硫酸盐氧化过程中的主要自由基种类。SO 4
-被认为是在pH主要自由基= 2两者SO 4
-和HO
促成DEP降解在pH = 7和HO
估计根据双C到具有21-63%贡献
H同位素分馏值。碳和氢的表观动力学同位素效应(AKIEs)(13 C-秋江= 1.017,2 H-秋江= 2.41)从主导DEP的硫酸根反应双方都支持C的假设得到的
H键裂解。因此,碳和氢同位素富集因子清楚地区分了不同的反应机理,因此,是一种有前途的方法,可以改善对PAEs化学氧化的自由基反应路径的了解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号