当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bismuth(iii)-catalyzed cycloisomerization and (hetero)arylation of alkynols: simple access to 2-(hetero)aryl tetrahydrofurans and tetrahydropyrans†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1039/c8ob00368h Ashwini K. Nakate 1, 2, 3, 4, 5 , Madhukar S. Pratapure 1, 2, 3, 4, 5 , Ravindar Kontham 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1039/c8ob00368h Ashwini K. Nakate 1, 2, 3, 4, 5 , Madhukar S. Pratapure 1, 2, 3, 4, 5 , Ravindar Kontham 1, 2, 3, 4, 5
Affiliation
|
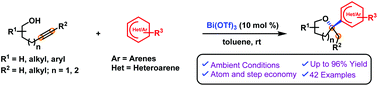
2-(Hetero)aryl tetrahydrofurans and tetrahydropyrans were successfully synthesized using Bi(OTf)3-catalyzed hydroalkoxylation (cycloisomerization) of alkynols (via 5 or 6 exo-dig cyclization) and intermolecular (hetero)arylation. This reaction involves a highly efficient cascade process, where initially the alkynol undergoes a cycloisomerization step via activation of the triple bond and generates the oxocarbenium ion, which subsequently participates in the (hetero)hydroarylation step with electron-rich arenes. Simple to complex suitably functionalized alkynols (4-pentyn-1-ols and 5-hexyn-1-ols) and electron-rich aromatic compounds were found to be reliable substrates in this cascade transformation and furnished a wide range of oxygen heterocycles. This practical tandem process provides a means to build libraries related to pharmacologically active molecules and natural product like scaffolds.
中文翻译:

铋(iii)催化的炔醇的环异构化和(杂)芳基化:可轻松获得2-(杂)芳基四氢呋喃和四氢吡喃†
使用Bi(OTf)3催化的炔醇加氢烷氧基化(环异构化)(通过5或6个exo- dig环化)和分子间(杂)芳基化反应成功合成了2-(杂)芳基四氢呋喃和四氢吡喃。该反应涉及高效的级联过程,其中炔醇首先通过以下步骤进行环异构化步骤激活三键并生成氧碳鎓离子,该氧碳鎓离子随后与富含电子的芳烃一起参与(杂)氢芳基化步骤。发现简单复杂的适当官能化的炔醇(4-戊炔-1-醇和5-己炔-1-醇)和富电子芳族化合物是这种级联转化的可靠底物,并提供了广泛的氧杂环。这种实用的串联过程提供了一种构建与药理活性分子和天然产物(如支架)有关的文库的方法。
更新日期:2018-04-06
中文翻译:

铋(iii)催化的炔醇的环异构化和(杂)芳基化:可轻松获得2-(杂)芳基四氢呋喃和四氢吡喃†
使用Bi(OTf)3催化的炔醇加氢烷氧基化(环异构化)(通过5或6个exo- dig环化)和分子间(杂)芳基化反应成功合成了2-(杂)芳基四氢呋喃和四氢吡喃。该反应涉及高效的级联过程,其中炔醇首先通过以下步骤进行环异构化步骤激活三键并生成氧碳鎓离子,该氧碳鎓离子随后与富含电子的芳烃一起参与(杂)氢芳基化步骤。发现简单复杂的适当官能化的炔醇(4-戊炔-1-醇和5-己炔-1-醇)和富电子芳族化合物是这种级联转化的可靠底物,并提供了广泛的氧杂环。这种实用的串联过程提供了一种构建与药理活性分子和天然产物(如支架)有关的文库的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号