Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2018-04-05 , DOI: 10.1007/s13361-018-1917-y Sibylle Pfammatter 1, 2 , Eric Bonneil 1 , Francis P. McManus 1 , Pierre Thibault 1, 2
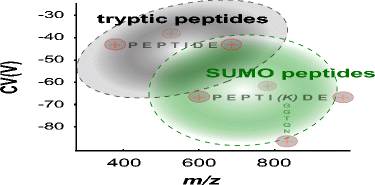
The small ubiquitin-like modifier (SUMO) is a member of the family of ubiquitin-like modifiers (UBLs) and is involved in important cellular processes, including DNA damage response, meiosis and cellular trafficking. The large-scale identification of SUMO peptides in a site-specific manner is challenging not only because of the low abundance and dynamic nature of this modification, but also due to the branched structure of the corresponding peptides that further complicate their identification using conventional search engines. Here, we exploited the unusual structure of SUMO peptides to facilitate their separation by high-field asymmetric waveform ion mobility spectrometry (FAIMS) and increase the coverage of SUMO proteome analysis. Upon trypsin digestion, branched peptides contain a SUMO remnant side chain and predominantly form triply protonated ions that facilitate their gas-phase separation using FAIMS. We evaluated the mobility characteristics of synthetic SUMO peptides and further demonstrated the application of FAIMS to profile the changes in protein SUMOylation of HEK293 cells following heat shock, a condition known to affect this modification. FAIMS typically provided a 10-fold improvement of detection limit of SUMO peptides, and enabled a 36% increase in SUMO proteome coverage compared to the same LC-MS/MS analyses performed without FAIMS.
ᅟ
中文翻译:

通过差分离子迁移率气相富集多个带电荷的肽离子,扩展了SUMO蛋白质组分析的全面性
小型泛素样修饰剂(SUMO)是泛素样修饰剂(UBL)家族的成员,参与重要的细胞过程,包括DNA损伤反应,减数分裂和细胞运输。SUMO肽以位点特异性的方式进行大规模鉴定不仅具有这种修饰的低丰度和动态特性,而且还由于相应肽的支链结构使使用常规搜索引擎进行鉴定变得更加复杂,因此具有挑战性。在这里,我们利用SUMO肽的异常结构,通过高场非对称波形离子迁移谱(FAIMS)促进了它们的分离,并增加了SUMO蛋白质组学分析的覆盖范围。胰蛋白酶消化后,支链肽含有一个SUMO残留侧链,并且主要形成三重质子化离子,有助于使用FAIMS进行气相分离。我们评估了合成SUMO肽的迁移特性,并进一步证明了FAIMS在热休克后(已知会影响这种修饰的条件)分析HEK293细胞蛋白质SUMO酰化变化的应用。与不使用FAIMS进行的相同LC-MS / MS分析相比,FAIMS通常可将SUMO肽的检测限提高10倍,并使SUMO蛋白质组覆盖率提高36%。我们评估了合成SUMO肽的迁移特性,并进一步证明了FAIMS在热休克后(已知会影响这种修饰的条件)分析HEK293细胞蛋白质SUMO酰化变化的应用。与不使用FAIMS进行的相同LC-MS / MS分析相比,FAIMS通常可将SUMO肽的检测限提高10倍,并使SUMO蛋白质组覆盖率提高36%。我们评估了合成SUMO肽的迁移特性,并进一步证明了FAIMS在热休克后(已知会影响这种修饰的条件)分析HEK293细胞蛋白质SUMO酰化变化的应用。与不使用FAIMS进行的相同LC-MS / MS分析相比,FAIMS通常可将SUMO肽的检测限提高10倍,并使SUMO蛋白质组覆盖率提高36%。

ᅟ


























 京公网安备 11010802027423号
京公网安备 11010802027423号