当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reversal of Ovarian Cancer Multidrug Resistance by a Combination of LAH4-L1-siMDR1 Nanocomplexes with Chemotherapeutics
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00031 Nana Guo 1, 2, 3 , Chen Gao 4 , Jing Liu 1 , Jun Li 5 , Nan Liu 6 , Yanli Hao 6 , Lei Chen 2, 3 , Xiaoning Zhang 1, 6
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00031 Nana Guo 1, 2, 3 , Chen Gao 4 , Jing Liu 1 , Jun Li 5 , Nan Liu 6 , Yanli Hao 6 , Lei Chen 2, 3 , Xiaoning Zhang 1, 6
Affiliation
|
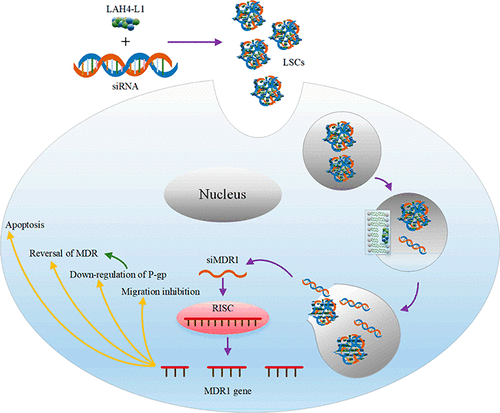
The mortality of ovarian cancer stably ranks first in gynecological malignancies due to the lack of specific symptoms and diagnostic methods at an early stage. For most patients, the cancer cells had metastasized before they were diagnosed. As a result, 90% of them died of multidrug resistance (MDR) to chemotherapeutics. In our study, RNAi technology was introduced and applied to overcome this big problem. LAH4-L1, an amphipathic cationic polypeptide, was reported to have high transfection efficiency and was first selected by us to deliver siMDR1 for overcoming ovarian cancer cells MDR. In this research, LAH4-L1-siRNA nanocomplexes (LSCs) delivery system was designed via electrostatic interactions. The nanocomplexes could realize 87.3% MDR1 gene silence and 85% P-gp down-regulation on SKOV-3 cells. What’s more, with the combination of chemotherapeutics, SKOV-3 cells growth inhibition can reach to 82.9%. We have also found that there was about 50% reduction on cells migration when MDR1 gene was down-regulated. Besides what have been mentioned above, physicochemical characteristics, cytotoxicity, pH responsivity, cells cycle, cellular uptake, and endosomal escape abilities were also studied in this research. In conclusion, lower cytotoxicity, higher down-regulation of targeted gene, and great cell inhibition, when combined with chemotherapeutics, all show the great potential of LSCs for the reversal of multidrug resistance on SKOV-3 cells in the future.
中文翻译:

LAH4-L1-siMDR1纳米复合物与化学治疗药物联合逆转卵巢癌多药耐药性
由于早期缺乏特异性症状和诊断方法,卵巢癌的死亡率在妇科恶性肿瘤中稳定居首位。对于大多数患者,癌细胞在被诊断之前已经转移。结果,其中90%的人死于对化疗药物的多药耐药性(MDR)。在我们的研究中,引入了RNAi技术并将其应用于克服这一大问题。据报道,LAH4-L1是一种两亲性阳离子多肽,具有很高的转染效率,并且首先被我们选择提供siMDR1来克服卵巢癌细胞MDR。在这项研究中,通过静电相互作用设计了LAH4-L1-siRNA纳米复合物(LSCs)递送系统。纳米复合物可以在SKOV-3细胞上实现87.3%的MDR1基因沉默和85%的P-gp下调。更重要的是,与化学疗法相结合,SKOV-3细胞的生长抑制率可达到82.9%。我们还发现当下调MDR1基因时,细胞迁移减少了约50%。除上述内容外,本研究还研究了理化特性,细胞毒性,pH响应性,细胞周期,细胞摄取和内体逃逸能力。总之,与化学疗法结合使用时,较低的细胞毒性,较高的靶向基因下调和良好的细胞抑制作用,都显示出LSCs将来有潜力逆转SKOV-3细胞的多药耐药性。在这项研究中还研究了理化特性,细胞毒性,pH响应性,细胞周期,细胞摄取和内体逃逸能力。总之,与化学疗法结合使用时,较低的细胞毒性,较高的靶向基因下调和良好的细胞抑制作用,都显示出LSCs将来有潜力逆转SKOV-3细胞的多药耐药性。在这项研究中还研究了理化特性,细胞毒性,pH响应性,细胞周期,细胞摄取和内体逃逸能力。总之,与化学疗法结合使用时,较低的细胞毒性,较高的靶向基因下调和良好的细胞抑制作用,都显示出LSCs将来有潜力逆转SKOV-3细胞的多药耐药性。
更新日期:2018-04-05
中文翻译:

LAH4-L1-siMDR1纳米复合物与化学治疗药物联合逆转卵巢癌多药耐药性
由于早期缺乏特异性症状和诊断方法,卵巢癌的死亡率在妇科恶性肿瘤中稳定居首位。对于大多数患者,癌细胞在被诊断之前已经转移。结果,其中90%的人死于对化疗药物的多药耐药性(MDR)。在我们的研究中,引入了RNAi技术并将其应用于克服这一大问题。据报道,LAH4-L1是一种两亲性阳离子多肽,具有很高的转染效率,并且首先被我们选择提供siMDR1来克服卵巢癌细胞MDR。在这项研究中,通过静电相互作用设计了LAH4-L1-siRNA纳米复合物(LSCs)递送系统。纳米复合物可以在SKOV-3细胞上实现87.3%的MDR1基因沉默和85%的P-gp下调。更重要的是,与化学疗法相结合,SKOV-3细胞的生长抑制率可达到82.9%。我们还发现当下调MDR1基因时,细胞迁移减少了约50%。除上述内容外,本研究还研究了理化特性,细胞毒性,pH响应性,细胞周期,细胞摄取和内体逃逸能力。总之,与化学疗法结合使用时,较低的细胞毒性,较高的靶向基因下调和良好的细胞抑制作用,都显示出LSCs将来有潜力逆转SKOV-3细胞的多药耐药性。在这项研究中还研究了理化特性,细胞毒性,pH响应性,细胞周期,细胞摄取和内体逃逸能力。总之,与化学疗法结合使用时,较低的细胞毒性,较高的靶向基因下调和良好的细胞抑制作用,都显示出LSCs将来有潜力逆转SKOV-3细胞的多药耐药性。在这项研究中还研究了理化特性,细胞毒性,pH响应性,细胞周期,细胞摄取和内体逃逸能力。总之,与化学疗法结合使用时,较低的细胞毒性,较高的靶向基因下调和良好的细胞抑制作用,都显示出LSCs将来有潜力逆转SKOV-3细胞的多药耐药性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号