当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acidity and basicity interplay in amide and imide self-association†
Chemical Science ( IF 8.4 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c8sc01020j Wilmer E Vallejo Narváez 1 , Eddy I Jiménez 1 , Eduardo Romero-Montalvo 1 , Arturo Sauza-de la Vega 1 , Beatriz Quiroz-García 1 , Marcos Hernández-Rodríguez 1 , Tomás Rocha-Rinza 1
Chemical Science ( IF 8.4 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c8sc01020j Wilmer E Vallejo Narváez 1 , Eddy I Jiménez 1 , Eduardo Romero-Montalvo 1 , Arturo Sauza-de la Vega 1 , Beatriz Quiroz-García 1 , Marcos Hernández-Rodríguez 1 , Tomás Rocha-Rinza 1
Affiliation
|
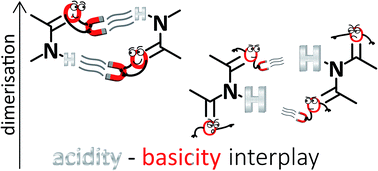
Amides dimerise more strongly than imides despite their lower acidity. Such an unexpected result has been rationalised in terms of the Jorgensen Secondary Interactions Hypothesis (JSIH) that involves the spectator (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OS) and H-bonded (C
OS) and H-bonded (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OHB) carbonyl groups in imides. Notwithstanding the considerable body of experimental and theoretical evidence supporting the JSIH, there are some computational studies which suggest that there might be other relevant intermolecular interactions than those considered in this model. We conjectured that the spectator carbonyl moieties could disrupt the resonance-assisted hydrogen bonds in imide dimers, but our results showed that this was not the case. Intrigued by this phenomenon, we studied the self-association of a set of amides and imides via1H-NMR, 1H-DOSY experiments, DFT calculations, QTAIM topological analyses of the electron density and IQA partitions of the electronic energy. These analyses revealed that there are indeed repulsions of the type OS⋯OHB in accordance with the JSIH but our data also indicate that the C
OHB) carbonyl groups in imides. Notwithstanding the considerable body of experimental and theoretical evidence supporting the JSIH, there are some computational studies which suggest that there might be other relevant intermolecular interactions than those considered in this model. We conjectured that the spectator carbonyl moieties could disrupt the resonance-assisted hydrogen bonds in imide dimers, but our results showed that this was not the case. Intrigued by this phenomenon, we studied the self-association of a set of amides and imides via1H-NMR, 1H-DOSY experiments, DFT calculations, QTAIM topological analyses of the electron density and IQA partitions of the electronic energy. These analyses revealed that there are indeed repulsions of the type OS⋯OHB in accordance with the JSIH but our data also indicate that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OS group has an overall attraction with the interacting molecule. Instead, we found correlations between self-association strength and simple Brønsted–Lowry acid/base properties, namely, N–H acidities and C
OS group has an overall attraction with the interacting molecule. Instead, we found correlations between self-association strength and simple Brønsted–Lowry acid/base properties, namely, N–H acidities and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O basicities. The results in CDCl3 and CCl4 indicate that imides dimerise less strongly than structurally related amides because of the lower basicity of their carbonyl fragments, a frequently overlooked aspect in the study of H-bonding. Overall, the model proposed herein could provide important insights in diverse areas of supramolecular chemistry such as the study of multiple hydrogen-bonded adducts which involve amide or imide functional groups.
O basicities. The results in CDCl3 and CCl4 indicate that imides dimerise less strongly than structurally related amides because of the lower basicity of their carbonyl fragments, a frequently overlooked aspect in the study of H-bonding. Overall, the model proposed herein could provide important insights in diverse areas of supramolecular chemistry such as the study of multiple hydrogen-bonded adducts which involve amide or imide functional groups.
中文翻译:

酰胺和酰亚胺自缔合中的酸碱相互作用†
尽管酸度较低,但酰胺比酰亚胺更强烈地二聚化。这种意想不到的结果已经在涉及旁观者(CO S)和氢键(CO HB)的约根森二次相互作用假设(JSIH)![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 方面得到了合理化
方面得到了合理化![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 。) 酰亚胺中的羰基。尽管有大量实验和理论证据支持 JSIH,但仍有一些计算研究表明,除了该模型中考虑的那些之外,可能还有其他相关的分子间相互作用。我们推测观察者羰基部分可以破坏酰亚胺二聚体中的共振辅助氢键,但我们的结果表明情况并非如此。受此现象的启发,我们通过1 H-NMR、1 H-DOSY 实验、DFT 计算、电子密度的 QTAIM 拓扑分析和电子能量的 IQA 分配研究了一组酰胺和酰亚胺的自缔合。这些分析表明确实存在 O S类型的排斥⋯O HB符合 JSIH 但我们的数据也表明 C
。) 酰亚胺中的羰基。尽管有大量实验和理论证据支持 JSIH,但仍有一些计算研究表明,除了该模型中考虑的那些之外,可能还有其他相关的分子间相互作用。我们推测观察者羰基部分可以破坏酰亚胺二聚体中的共振辅助氢键,但我们的结果表明情况并非如此。受此现象的启发,我们通过1 H-NMR、1 H-DOSY 实验、DFT 计算、电子密度的 QTAIM 拓扑分析和电子能量的 IQA 分配研究了一组酰胺和酰亚胺的自缔合。这些分析表明确实存在 O S类型的排斥⋯O HB符合 JSIH 但我们的数据也表明 C ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O S基团与相互作用的分子具有整体吸引力。相反,我们发现自缔合强度与简单的 Brønsted-Lowry 酸/碱性质(即 N-H 酸度和
O S基团与相互作用的分子具有整体吸引力。相反,我们发现自缔合强度与简单的 Brønsted-Lowry 酸/碱性质(即 N-H 酸度和![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO 碱度)之间存在相关性。CDCl 3和 CCl 4中的结果表明由于其羰基片段的碱度较低,酰亚胺的二聚化强度不如结构相关的酰胺,这是氢键研究中经常被忽视的一个方面。总体而言,本文提出的模型可以为超分子化学的不同领域提供重要的见解,例如研究涉及酰胺或酰亚胺官能团的多个氢键加合物。
CO 碱度)之间存在相关性。CDCl 3和 CCl 4中的结果表明由于其羰基片段的碱度较低,酰亚胺的二聚化强度不如结构相关的酰胺,这是氢键研究中经常被忽视的一个方面。总体而言,本文提出的模型可以为超分子化学的不同领域提供重要的见解,例如研究涉及酰胺或酰亚胺官能团的多个氢键加合物。
更新日期:2018-04-05
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OS) and H-bonded (C
OS) and H-bonded (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OHB) carbonyl groups in imides. Notwithstanding the considerable body of experimental and theoretical evidence supporting the JSIH, there are some computational studies which suggest that there might be other relevant intermolecular interactions than those considered in this model. We conjectured that the spectator carbonyl moieties could disrupt the resonance-assisted hydrogen bonds in imide dimers, but our results showed that this was not the case. Intrigued by this phenomenon, we studied the self-association of a set of amides and imides via1H-NMR, 1H-DOSY experiments, DFT calculations, QTAIM topological analyses of the electron density and IQA partitions of the electronic energy. These analyses revealed that there are indeed repulsions of the type OS⋯OHB in accordance with the JSIH but our data also indicate that the C
OHB) carbonyl groups in imides. Notwithstanding the considerable body of experimental and theoretical evidence supporting the JSIH, there are some computational studies which suggest that there might be other relevant intermolecular interactions than those considered in this model. We conjectured that the spectator carbonyl moieties could disrupt the resonance-assisted hydrogen bonds in imide dimers, but our results showed that this was not the case. Intrigued by this phenomenon, we studied the self-association of a set of amides and imides via1H-NMR, 1H-DOSY experiments, DFT calculations, QTAIM topological analyses of the electron density and IQA partitions of the electronic energy. These analyses revealed that there are indeed repulsions of the type OS⋯OHB in accordance with the JSIH but our data also indicate that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OS group has an overall attraction with the interacting molecule. Instead, we found correlations between self-association strength and simple Brønsted–Lowry acid/base properties, namely, N–H acidities and C
OS group has an overall attraction with the interacting molecule. Instead, we found correlations between self-association strength and simple Brønsted–Lowry acid/base properties, namely, N–H acidities and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O basicities. The results in CDCl3 and CCl4 indicate that imides dimerise less strongly than structurally related amides because of the lower basicity of their carbonyl fragments, a frequently overlooked aspect in the study of H-bonding. Overall, the model proposed herein could provide important insights in diverse areas of supramolecular chemistry such as the study of multiple hydrogen-bonded adducts which involve amide or imide functional groups.
O basicities. The results in CDCl3 and CCl4 indicate that imides dimerise less strongly than structurally related amides because of the lower basicity of their carbonyl fragments, a frequently overlooked aspect in the study of H-bonding. Overall, the model proposed herein could provide important insights in diverse areas of supramolecular chemistry such as the study of multiple hydrogen-bonded adducts which involve amide or imide functional groups.
中文翻译:

酰胺和酰亚胺自缔合中的酸碱相互作用†
尽管酸度较低,但酰胺比酰亚胺更强烈地二聚化。这种意想不到的结果已经在涉及旁观者(CO S)和氢键(CO HB)的约根森二次相互作用假设(JSIH)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 方面得到了合理化
方面得到了合理化![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 。) 酰亚胺中的羰基。尽管有大量实验和理论证据支持 JSIH,但仍有一些计算研究表明,除了该模型中考虑的那些之外,可能还有其他相关的分子间相互作用。我们推测观察者羰基部分可以破坏酰亚胺二聚体中的共振辅助氢键,但我们的结果表明情况并非如此。受此现象的启发,我们通过1 H-NMR、1 H-DOSY 实验、DFT 计算、电子密度的 QTAIM 拓扑分析和电子能量的 IQA 分配研究了一组酰胺和酰亚胺的自缔合。这些分析表明确实存在 O S类型的排斥⋯O HB符合 JSIH 但我们的数据也表明 C
。) 酰亚胺中的羰基。尽管有大量实验和理论证据支持 JSIH,但仍有一些计算研究表明,除了该模型中考虑的那些之外,可能还有其他相关的分子间相互作用。我们推测观察者羰基部分可以破坏酰亚胺二聚体中的共振辅助氢键,但我们的结果表明情况并非如此。受此现象的启发,我们通过1 H-NMR、1 H-DOSY 实验、DFT 计算、电子密度的 QTAIM 拓扑分析和电子能量的 IQA 分配研究了一组酰胺和酰亚胺的自缔合。这些分析表明确实存在 O S类型的排斥⋯O HB符合 JSIH 但我们的数据也表明 C ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O S基团与相互作用的分子具有整体吸引力。相反,我们发现自缔合强度与简单的 Brønsted-Lowry 酸/碱性质(即 N-H 酸度和
O S基团与相互作用的分子具有整体吸引力。相反,我们发现自缔合强度与简单的 Brønsted-Lowry 酸/碱性质(即 N-H 酸度和![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO 碱度)之间存在相关性。CDCl 3和 CCl 4中的结果表明由于其羰基片段的碱度较低,酰亚胺的二聚化强度不如结构相关的酰胺,这是氢键研究中经常被忽视的一个方面。总体而言,本文提出的模型可以为超分子化学的不同领域提供重要的见解,例如研究涉及酰胺或酰亚胺官能团的多个氢键加合物。
CO 碱度)之间存在相关性。CDCl 3和 CCl 4中的结果表明由于其羰基片段的碱度较低,酰亚胺的二聚化强度不如结构相关的酰胺,这是氢键研究中经常被忽视的一个方面。总体而言,本文提出的模型可以为超分子化学的不同领域提供重要的见解,例如研究涉及酰胺或酰亚胺官能团的多个氢键加合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号