Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the Blastochloris viridis LH1–RC complex at 2.9 Å
Nature ( IF 64.8 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0014-5 Pu Qian , C. Alistair Siebert , Peiyi Wang , Daniel P. Canniffe , C. Neil Hunter
Nature ( IF 64.8 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0014-5 Pu Qian , C. Alistair Siebert , Peiyi Wang , Daniel P. Canniffe , C. Neil Hunter
|
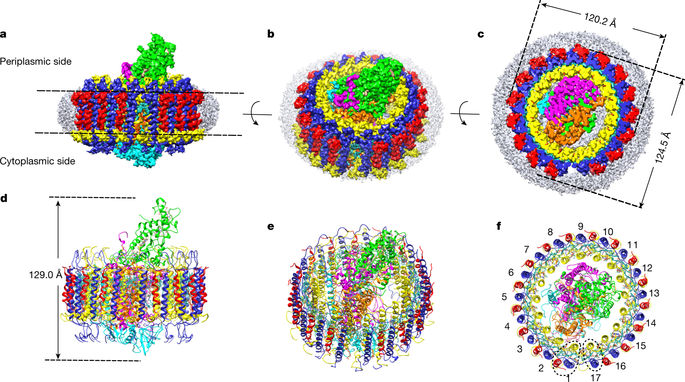
AbstractThe light-harvesting 1–reaction centre (LH1–RC) complex is a key functional component of bacterial photosynthesis. Here we present a 2.9 Å resolution cryo-electron microscopy structure of the bacteriochlorophyll b-based LH1–RC complex from Blastochloris viridis that reveals the structural basis for absorption of infrared light and the molecular mechanism of quinone migration across the LH1 complex. The triple-ring LH1 complex comprises a circular array of 17 β-polypeptides sandwiched between 17 α- and 16 γ-polypeptides. Tight packing of the γ-apoproteins between β-polypeptides collectively interlocks and stabilizes the LH1 structure; this, together with the short Mg–Mg distances of bacteriochlorophyll b pairs, contributes to the large redshift of bacteriochlorophyll b absorption. The ‘missing’ 17th γ-polypeptide creates a pore in the LH1 ring, and an adjacent binding pocket provides a folding template for a quinone, Q P, which adopts a compact, export-ready conformation before passage through the pore and eventual diffusion to the cytochrome bc1 complex.A cryo-electron microscopy structure of the light-harvesting–reaction centre (LH1–RC) complex of the photosynthetic bacterium Blastochloris viridis suggests factors that underlie the large redshift in the absorption spectrum of bacteriochlorophyll in the complex and that promote quinone–quinol translocation across the LH1 ring.
中文翻译:

Blastochloris viridis LH1-RC 复合物在 2.9 Å 处的冷冻电镜结构
摘要 捕光 1-反应中心 (LH1-RC) 复合物是细菌光合作用的关键功能成分。在这里,我们展示了来自绿色芽孢杆菌的基于细菌叶绿素 b 的 LH1-RC 复合物的 2.9 Å 分辨率冷冻电子显微镜结构,揭示了吸收红外光的结构基础和醌迁移穿过 LH1 复合物的分子机制。三环 LH1 复合物包含 17 个 β-多肽的圆形阵列,夹在 17 个 α-和 16 个 γ-多肽之间。β-多肽之间的 γ-载脂蛋白的紧密堆积共同连锁并稳定 LH1 结构;这与细菌叶绿素 b 对的短 Mg-Mg 距离一起,导致了细菌叶绿素 b 吸收的大红移。
更新日期:2018-04-01
中文翻译:

Blastochloris viridis LH1-RC 复合物在 2.9 Å 处的冷冻电镜结构
摘要 捕光 1-反应中心 (LH1-RC) 复合物是细菌光合作用的关键功能成分。在这里,我们展示了来自绿色芽孢杆菌的基于细菌叶绿素 b 的 LH1-RC 复合物的 2.9 Å 分辨率冷冻电子显微镜结构,揭示了吸收红外光的结构基础和醌迁移穿过 LH1 复合物的分子机制。三环 LH1 复合物包含 17 个 β-多肽的圆形阵列,夹在 17 个 α-和 16 个 γ-多肽之间。β-多肽之间的 γ-载脂蛋白的紧密堆积共同连锁并稳定 LH1 结构;这与细菌叶绿素 b 对的短 Mg-Mg 距离一起,导致了细菌叶绿素 b 吸收的大红移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号