当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic studies on the N-alkylation of amines with alcohols catalysed by iridium(i) complexes with functionalised N-heterocyclic carbene ligands†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c7cy02488f M. Victoria Jiménez 1, 2, 3, 4, 5 , Javier Fernández-Tornos 1, 2, 3, 4, 5 , Miguel González-Lainez 1, 2, 3, 4, 5 , Beatriz Sánchez-Page 1, 2, 3, 4, 5 , F. Javier Modrego 1, 2, 3, 4, 5 , Luis A. Oro 1, 2, 3, 4, 5 , Jesús J. Pérez-Torrente 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c7cy02488f M. Victoria Jiménez 1, 2, 3, 4, 5 , Javier Fernández-Tornos 1, 2, 3, 4, 5 , Miguel González-Lainez 1, 2, 3, 4, 5 , Beatriz Sánchez-Page 1, 2, 3, 4, 5 , F. Javier Modrego 1, 2, 3, 4, 5 , Luis A. Oro 1, 2, 3, 4, 5 , Jesús J. Pérez-Torrente 1, 2, 3, 4, 5
Affiliation
|
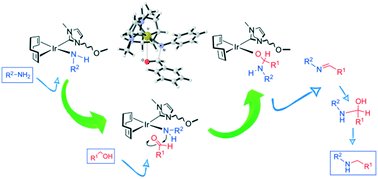
Iridium(I) cyclooctadiene complexes featuring O- and N-donor functionalised NHC ligands efficiently catalyse the C–N coupling of amines with alcohols through a borrowing hydrogen mechanism. These catalysts have been applied for the N-alkylation of several aromatic and aliphatic primary amines with a range of alcohols including benzyl alcohol derivatives, straight-chain primary alcohols and secondary alcohols. The cationic complex [Ir(NCCH3)(cod){MeIm(2-methoxybenzyl)}]+ (cod = 1,5-cyclooctadiene, MeIm = 3-methylimidazol-2-ylidene) having a rigid O-donor wingtip exhibits the best catalytic performance for the N-alkylation of aniline with benzyl alcohol giving a quantitative conversion to N-benzylaniline in 3 h. Experimental and theoretical studies at the DFT level on the N-alkylation of aniline with benzyl alcohol catalysed by the model compound [IrCl(cod)(IMe)] (IMe = 1,3-dimethyl-imidazol-2-ylidene) support the participation of the iridium catalyst not only in the alcohol dehydrogenation and imine hydrogenation steps but also in the key step leading to the formation of the new C–N bond. Nucleophilic attack of an iridium-amido species generated in basic medium on the electrophilic aldehyde results in a hemiaminolate intermediate species from which the hemiaminal is released by alcoholysis. The free hemiaminal dehydrates to give the corresponding intermediate imine product that is hydrogenated by the iridium catalyst to the N-alkylated amine product. The iridium(I) complexes featuring functionalised NHC ligands are more active than [IrCl(cod)(IMe)] which highlights the positive influence of the functional group on the N-alkylation catalytic activity.
中文翻译:

上的机制研究Ñ胺的烷基化通过铱催化的醇(我)与官能化的N-杂环卡宾配体的络合物†
具有O和N供体官能化的NHC配体的铱(I)环辛二烯络合物通过借用的氢机制有效催化胺与醇的C–N偶联。这些催化剂已经用于几种芳族和脂族伯胺与一系列醇的N-烷基化,所述醇包括苄醇衍生物,直链伯醇和仲醇。具有刚性O-给体翼尖的阳离子络合物[Ir(NCCH 3)(cod){MeIm(2-甲氧基苄基)}] +(cod = 1,5-环辛二烯,MeIm = 3-甲基咪唑-2-亚烷基)表现出苯胺与苯甲醇的N-烷基化反应的最佳催化性能,可定量转化为N-苄基苯胺在3小时内。在模型化合物[IrCl(cod)(IMe)](IMe = 1,3-二甲基-咪唑-2-亚烷基)催化下,在苯胺与苯甲醇的N-烷基化的DFT级实验和理论研究支持该研究铱催化剂的制备不仅在醇脱氢和亚胺加氢步骤中,而且在导致形成新的C–N键的关键步骤中也是如此。在碱性介质中产生的铱-氨基物种对亲电子醛的亲核攻击导致形成半氨基化物中间物种,通过醇解作用从中释放半胱氨酸。游离的羟胺脱水,得到相应的中间体亚胺产物,该产物由铱催化剂氢化为N-烷基化的胺产物。铱(I)具有官能化NHC配体的配合物比[IrCl(cod)(IMe)]更具活性,这突出了官能团对N-烷基化催化活性的积极影响。
更新日期:2018-04-02
中文翻译:

上的机制研究Ñ胺的烷基化通过铱催化的醇(我)与官能化的N-杂环卡宾配体的络合物†
具有O和N供体官能化的NHC配体的铱(I)环辛二烯络合物通过借用的氢机制有效催化胺与醇的C–N偶联。这些催化剂已经用于几种芳族和脂族伯胺与一系列醇的N-烷基化,所述醇包括苄醇衍生物,直链伯醇和仲醇。具有刚性O-给体翼尖的阳离子络合物[Ir(NCCH 3)(cod){MeIm(2-甲氧基苄基)}] +(cod = 1,5-环辛二烯,MeIm = 3-甲基咪唑-2-亚烷基)表现出苯胺与苯甲醇的N-烷基化反应的最佳催化性能,可定量转化为N-苄基苯胺在3小时内。在模型化合物[IrCl(cod)(IMe)](IMe = 1,3-二甲基-咪唑-2-亚烷基)催化下,在苯胺与苯甲醇的N-烷基化的DFT级实验和理论研究支持该研究铱催化剂的制备不仅在醇脱氢和亚胺加氢步骤中,而且在导致形成新的C–N键的关键步骤中也是如此。在碱性介质中产生的铱-氨基物种对亲电子醛的亲核攻击导致形成半氨基化物中间物种,通过醇解作用从中释放半胱氨酸。游离的羟胺脱水,得到相应的中间体亚胺产物,该产物由铱催化剂氢化为N-烷基化的胺产物。铱(I)具有官能化NHC配体的配合物比[IrCl(cod)(IMe)]更具活性,这突出了官能团对N-烷基化催化活性的积极影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号