当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
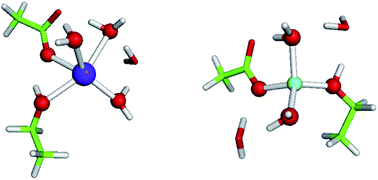
Competition between Li+ and Na+ in sodium transporters and receptors: Which Na+-Binding sites are “therapeutic” Li+ targets?†
Chemical Science ( IF 8.4 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c7sc05284g Todor Dudev,Karine Mazmanian,Carmay Lim
Chemical Science ( IF 8.4 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c7sc05284g Todor Dudev,Karine Mazmanian,Carmay Lim
|
Sodium (Na+) acts as an indispensable allosteric regulator of the activities of biologically important neurotransmitter transporters and G-protein coupled receptors (GPCRs), which comprise well-known drug targets for psychiatric disorders and addictive behavior. How selective these allosteric Na+-binding sites are for the cognate cation over abiogenic Li+, a first-line drug to treat bipolar disorder, is unclear. Here, we reveal how properties of the host protein and its binding cavity affect the outcome of the competition between Li+ and Na+ for allosteric binding sites in sodium transporters and receptors. We show that rigid Na+-sites that are crowded with multiple protein ligands are well-protected against Li+ attack, but their flexible counterparts or buried Na+-sites containing only one or two protein ligands are vulnerable to Li+ substitution. These findings suggest a novel possible mode of Li+ therapeutic action: By displacing Na+ bound by ≤2 protein ligands in buried GPCR sites and stabilizing the receptor's inactive state, Li+ could prohibit conformational changes to an active state, leading to lower cytosolic levels of activated guanine nucleotide-binding proteins, which are hyperactive/overexpressed in bipolar disorder patients.
中文翻译:

Li +和Na +在钠转运蛋白和受体之间的竞争:哪些Na +结合位点是“治疗性” Li +靶标?†
钠(Na +)是生物学上重要的神经递质转运蛋白和G蛋白偶联受体(GPCR)的活动的必不可少的变构调节剂,GPCR包括精神疾病和成瘾行为的知名药物靶标。目前尚不清楚这些变构Na +结合位点相对于非生物成因Li +(治疗双相情感障碍的一线药物)对同源阳离子的选择性如何。在这里,我们揭示了宿主蛋白及其结合腔的特性如何影响钠转运蛋白和受体中变构结合位点的Li +和Na +之间竞争的结果。我们显示出刚性的Na +拥挤有多种蛋白质配体的位点可以很好地抵抗Li +攻击,但它们的柔性对应位点或仅包含一个或两个蛋白质配体的Na +掩埋位点易受Li +取代。这些发现暗示了Li +治疗作用的新模式:通过置换埋藏在GPCR位点中≤2个蛋白质配体的Na +并稳定受体的非活性状态,Li +可以阻止构象改变为活性状态,从而降低胞浆水平激活的鸟嘌呤核苷酸结合蛋白,在双相情感障碍患者中过度活跃/过表达。
更新日期:2018-04-02
中文翻译:

Li +和Na +在钠转运蛋白和受体之间的竞争:哪些Na +结合位点是“治疗性” Li +靶标?†
钠(Na +)是生物学上重要的神经递质转运蛋白和G蛋白偶联受体(GPCR)的活动的必不可少的变构调节剂,GPCR包括精神疾病和成瘾行为的知名药物靶标。目前尚不清楚这些变构Na +结合位点相对于非生物成因Li +(治疗双相情感障碍的一线药物)对同源阳离子的选择性如何。在这里,我们揭示了宿主蛋白及其结合腔的特性如何影响钠转运蛋白和受体中变构结合位点的Li +和Na +之间竞争的结果。我们显示出刚性的Na +拥挤有多种蛋白质配体的位点可以很好地抵抗Li +攻击,但它们的柔性对应位点或仅包含一个或两个蛋白质配体的Na +掩埋位点易受Li +取代。这些发现暗示了Li +治疗作用的新模式:通过置换埋藏在GPCR位点中≤2个蛋白质配体的Na +并稳定受体的非活性状态,Li +可以阻止构象改变为活性状态,从而降低胞浆水平激活的鸟嘌呤核苷酸结合蛋白,在双相情感障碍患者中过度活跃/过表达。



























 京公网安备 11010802027423号
京公网安备 11010802027423号