当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
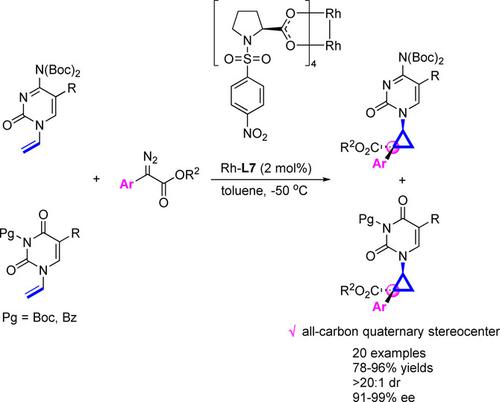
Construction of All‐Carbon Quaternary Stereocenters via Asymmetric Cyclopropanations: Synthesis of Chiral Carbocyclic Pyrimidine Nucleosides
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-04-20 , DOI: 10.1002/adsc.201800222 Hai-Xia Wang 1 , Fang-Juan Guan 1 , Ming-Sheng Xie 1 , Gui-Rong Qu 1 , Hai-Ming Guo 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-04-20 , DOI: 10.1002/adsc.201800222 Hai-Xia Wang 1 , Fang-Juan Guan 1 , Ming-Sheng Xie 1 , Gui-Rong Qu 1 , Hai-Ming Guo 1
Affiliation
|
An efficient route to synthesize chiral carbocyclic pyrimidine nucleoside analogues containing all‐carbon quaternary stereocenters has been established via the asymmetric intermolecular cyclopropanation of N1‐vinylpyrimidines and α‐aryl diazoesters. With 2 mol% of chiral dirhodium (II) carboxylate complex as the catalyst, a variety of chiral carbocyclic cytosine or uracil nucleoside analogues were obtained in good yields (up to 96% yield), high diastereoselectivities (>20:1 dr), and excellent enantioselectivities (up to 99% ee).
中文翻译:

通过不对称环丙烷化构建全碳四元立体中心:手性碳环嘧啶核苷的合成
通过N1-乙烯基嘧啶和α-芳基重氮酸酯的不对称分子间环丙烷化,已经建立了一种有效的途径来合成包含全碳四级立体中心的手性碳环嘧啶核苷类似物。以2摩尔%的手性羧酸二氢吡啶鎓(II)络合物为催化剂,可以以高收率(高达96%收率),高非对映选择性(> 20:1 dr)和高收率获得各种手性碳环胞嘧啶或尿嘧啶核苷类似物。极好的对映选择性(高达99%ee)。
更新日期:2018-04-20
中文翻译:

通过不对称环丙烷化构建全碳四元立体中心:手性碳环嘧啶核苷的合成
通过N1-乙烯基嘧啶和α-芳基重氮酸酯的不对称分子间环丙烷化,已经建立了一种有效的途径来合成包含全碳四级立体中心的手性碳环嘧啶核苷类似物。以2摩尔%的手性羧酸二氢吡啶鎓(II)络合物为催化剂,可以以高收率(高达96%收率),高非对映选择性(> 20:1 dr)和高收率获得各种手性碳环胞嘧啶或尿嘧啶核苷类似物。极好的对映选择性(高达99%ee)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号